Dissecting the molecular assembly of the Toxoplasma gondii MyoA motility complex
- PMID: 28972141
- PMCID: PMC5702683
- DOI: 10.1074/jbc.M117.809632
Dissecting the molecular assembly of the Toxoplasma gondii MyoA motility complex
Abstract
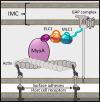
Apicomplexan parasites such as Toxoplasma gondii rely on a unique form of locomotion known as gliding motility. Generating the mechanical forces to support motility are divergent class XIV myosins (MyoA) coordinated by accessory proteins known as light chains. Although the importance of the MyoA-light chain complex is well-established, the detailed mechanisms governing its assembly and regulation are relatively unknown. To establish a molecular blueprint of this dynamic complex, we first mapped the adjacent binding sites of light chains MLC1 and ELC1 on the MyoA neck (residues 775-818) using a combination of hydrogen-deuterium exchange mass spectrometry and isothermal titration calorimetry. We then determined the 1.85 Å resolution crystal structure of MLC1 in complex with its cognate MyoA peptide. Structural analysis revealed a bilobed architecture with MLC1 clamping tightly around the helical MyoA peptide, consistent with the stable 10 nm Kd measured by isothermal titration calorimetry. We next showed that coordination of calcium by an EF-hand in ELC1 and prebinding of MLC1 to the MyoA neck enhanced the affinity of ELC1 for the MyoA neck 7- and 8-fold, respectively. When combined, these factors enhanced ELC1 binding 49-fold (to a Kd of 12 nm). Using the full-length MyoA motor (residues 1-831), we then showed that, in addition to coordinating the neck region, ELC1 appears to engage the MyoA converter subdomain, which couples the motor domain to the neck. These data support an assembly model where staged binding events cooperate to yield high-affinity complexes that are able to maximize force transduction.
Keywords: Toxoplasma gondii; X-ray crystallography; cell motility; crystal structure; host cell invasion; isothermal titration calorimetry (ITC); myosin; protein structure.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


Similar articles
-
Structural and mechanistic insights into the function of the unconventional class XIV myosin MyoA from Toxoplasma gondii.Proc Natl Acad Sci U S A. 2018 Nov 6;115(45):E10548-E10555. doi: 10.1073/pnas.1811167115. Epub 2018 Oct 22. Proc Natl Acad Sci U S A. 2018. PMID: 30348763 Free PMC article.
-
Two Essential Light Chains Regulate the MyoA Lever Arm To Promote Toxoplasma Gliding Motility.mBio. 2015 Sep 15;6(5):e00845-15. doi: 10.1128/mBio.00845-15. mBio. 2015. PMID: 26374117 Free PMC article.
-
The toxoplasma Acto-MyoA motor complex is important but not essential for gliding motility and host cell invasion.PLoS One. 2014 Mar 14;9(3):e91819. doi: 10.1371/journal.pone.0091819. eCollection 2014. PLoS One. 2014. PMID: 24632839 Free PMC article.
-
Environmental sensing and regulation of motility in Toxoplasma.Mol Microbiol. 2021 May;115(5):916-929. doi: 10.1111/mmi.14661. Epub 2020 Dec 28. Mol Microbiol. 2021. PMID: 33278047 Review.
-
Microneme proteins: structural and functional requirements to promote adhesion and invasion by the apicomplexan parasite Toxoplasma gondii.Int J Parasitol. 2001 Oct;31(12):1293-302. doi: 10.1016/s0020-7519(01)00257-0. Int J Parasitol. 2001. PMID: 11566297 Review.
Cited by
-
Dynamic structural biology at the protein membrane interface.J Biol Chem. 2019 Mar 15;294(11):3872-3880. doi: 10.1074/jbc.AW118.003236. Epub 2019 Jan 28. J Biol Chem. 2019. PMID: 30692197 Free PMC article. Review.
-
Functional characterization of calmodulin-like proteins, CML13 and CML14, as novel light chains of Arabidopsis class VIII myosins.J Exp Bot. 2024 Apr 15;75(8):2313-2329. doi: 10.1093/jxb/erae031. J Exp Bot. 2024. PMID: 38280207 Free PMC article.
-
Myosin A and F-Actin play a critical role in mitochondrial dynamics and inheritance in Toxoplasma gondii.bioRxiv [Preprint]. 2024 Mar 18:2024.03.18.585462. doi: 10.1101/2024.03.18.585462. bioRxiv. 2024. Update in: PLoS Pathog. 2024 Oct 7;20(10):e1012127. doi: 10.1371/journal.ppat.1012127. PMID: 38562694 Free PMC article. Updated. Preprint.
-
Myosin A and F-Actin play a critical role in mitochondrial dynamics and inheritance in Toxoplasma gondii.PLoS Pathog. 2024 Oct 7;20(10):e1012127. doi: 10.1371/journal.ppat.1012127. eCollection 2024 Oct. PLoS Pathog. 2024. PMID: 39374269 Free PMC article.
-
Deletion of splicing factor Cdc5 in Toxoplasma disrupts transcriptome integrity, induces abortive bradyzoite formation, and prevents acute infection in mice.Nat Commun. 2025 Apr 22;16(1):3769. doi: 10.1038/s41467-025-58805-3. Nat Commun. 2025. PMID: 40263328 Free PMC article.
References
-
- World Health Organization (2015) Guidelines for the treatment of malaria, 3rd Ed., World Health Organization Press, Geneva, Switzerland
-
- Checkley W., White A. C. Jr., Jaganath D., Arrowood M. J., Chalmers R. M., Chen X. M., Fayer R., Griffiths J. K., Guerrant R. L., Hedstrom L., Huston C. D., Kotloff K. L., Kang G., Mead J. R., Miller M., et al. (2015) A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. Lancet Infect. Dis. 15, 85–94 - PMC - PubMed
-
- Luft B. J., and Remington J. S. (1992) Toxoplasmic encephalitis in AIDS. Clin. Infect. Dis. 15, 211–222 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

