Structural analyses of the MazEF4 toxin-antitoxin pair in Mycobacterium tuberculosis provide evidence for a unique extracellular death factor
- PMID: 28972145
- PMCID: PMC5704469
- DOI: 10.1074/jbc.M117.807974
Structural analyses of the MazEF4 toxin-antitoxin pair in Mycobacterium tuberculosis provide evidence for a unique extracellular death factor
Abstract
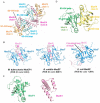
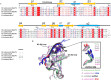
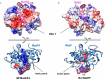
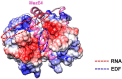
The bacterial toxin-antitoxin MazEF system in the tuberculosis (TB)-causing bacterium Mycobacterium tuberculosis is activated under unfavorable conditions, including starvation, antibiotic exposure, and oxidative stress. This system contains the ribonucleolytic enzyme MazF and has emerged as a promising drug target for TB treatments targeting the latent stage of M. tuberculosis infection and reportedly mediates a cell death process via a peptide called extracellular death factor (EDF). Although it is well established that the increase in EDF-mediated toxicity of MazF drives a cell-killing phenomenon, the molecular details are poorly understood. Moreover, the divergence in sequences among reported EDFs suggests that each bacterial species has a unique EDF. To address these open questions, we report here the structures of MazF4 and MazEF4 complexes from M. tuberculosis, representing the first MazEF structures from this organism. We found that MazF4 possesses a negatively charged MazE4-binding pocket in contrast to the positively charged MazE-binding pockets in homologous MazEF complex structures from other bacteria. Moreover, using NMR spectroscopy and biochemical assays, we unraveled the molecular interactions of MazF4 with its RNA substrate and with a new EDF homolog originating from M. tuberculosis The EDF homolog discovered here possesses a positively charged residue at the C terminus, making this EDF distinct from previously reported EDFs. Overall, our results suggest that M. tuberculosis evolved a unique MazF and EDF and that the distinctive EDF sequence could serve as a starting point for designing new anti-tuberculosis drugs. We therefore conclude that this study might contribute to the development of a new line of anti-tuberculosis agents.
Keywords: MazEF system; Mycobacterium tuberculosis; X-ray crystallography; extracellular death factor; nuclear magnetic resonance (NMR); protein structure; quorum sensing; toxin-antitoxin system.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Lönnroth K., and Raviglione M. (2008) Global epidemiology of tuberculosis: prospects for control. Semin. Respir. Crit. Care Med. 29, 481–491 - PubMed
-
- WHO. (2016) Global Tuberculosis Report 2016. World Health Organization, Geneva, Switzerland
-
- Schluger N. W. (2013) Advances in the diagnosis of latent tuberculosis infection. Semin. Respir. Crit. Care Med. 34, 60–66 - PubMed
-
- Hayes F., and Van Melderen L. (2011) Toxins-antitoxins: diversity, evolution and function. Crit. Rev. Biochem. Mol. Biol. 46, 386–408 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

