Defining a mechanistic link between pigment epithelium-derived factor, docosahexaenoic acid, and corneal nerve regeneration
- PMID: 28972155
- PMCID: PMC5682960
- DOI: 10.1074/jbc.M117.801472
Defining a mechanistic link between pigment epithelium-derived factor, docosahexaenoic acid, and corneal nerve regeneration
Abstract
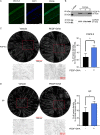
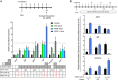
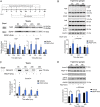
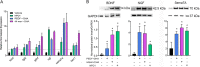
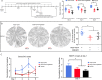
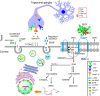
The cornea is densely innervated to sustain the integrity of the ocular surface. Corneal nerve damage produced by aging, diabetes, refractive surgeries, and viral or bacterial infections impairs tear production, the blinking reflex, and epithelial wound healing, resulting in loss of transparency and vision. A combination of the known neuroprotective molecule, pigment epithelium-derived factor (PEDF) plus docosahexaenoic acid (DHA), has been shown to stimulate corneal nerve regeneration, but the mechanisms involved are unclear. Here, we sought to define the molecular events of this effect in an in vivo mouse injury model. We first confirmed that PEDF + DHA increased nerve regeneration in the mouse cornea. Treatment with PEDF activates the phospholipase A2 activity of the PEDF-receptor (PEDF-R) leading to the release of DHA; this free DHA led to enhanced docosanoid synthesis and induction of bdnf, ngf, and the axon growth promoter semaphorin 7a (sema7a), and as a consequence, their products appeared in the mouse tears. Surprisingly, corneal injury and treatment with PEDF + DHA induced transcription of neuropeptide y (npy), small proline-rich protein 1a (sprr1a), and vasoactive intestinal peptide (vip) in the trigeminal ganglia (TG). The PEDF-R inhibitor, atglistatin, blocked all of these changes in the cornea and TG. In conclusion, we uncovered here an active cornea-TG axis, driven by PEDF-R activation, that fosters axon outgrowth in the cornea.
Keywords: DHA; NGF; PEDF; Sema7A; adipose triglyceride lipase; brain-derived neurotrophic factor (BDNF); cornea; lipid signaling; neuropeptides; phospholipase A.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

Similar articles
-
PEDF plus DHA modulate inflammation and stimulate nerve regeneration after HSV-1 infection.Exp Eye Res. 2017 Aug;161:153-162. doi: 10.1016/j.exer.2017.06.015. Epub 2017 Jun 20. Exp Eye Res. 2017. PMID: 28642110 Free PMC article.
-
Neuroprotectin D1 synthesis and corneal nerve regeneration after experimental surgery and treatment with PEDF plus DHA.Invest Ophthalmol Vis Sci. 2010 Feb;51(2):804-10. doi: 10.1167/iovs.09-3641. Epub 2009 Sep 24. Invest Ophthalmol Vis Sci. 2010. PMID: 19797230 Free PMC article.
-
Recovery of Corneal Sensitivity and Increase in Nerve Density and Wound Healing in Diabetic Mice After PEDF Plus DHA Treatment.Diabetes. 2017 Sep;66(9):2511-2520. doi: 10.2337/db17-0249. Epub 2017 Jun 7. Diabetes. 2017. PMID: 28592408 Free PMC article.
-
A new R,R-RvD6 isomer with protective actions following corneal nerve injury.Prostaglandins Other Lipid Mediat. 2024 Feb;170:106802. doi: 10.1016/j.prostaglandins.2023.106802. Epub 2023 Nov 29. Prostaglandins Other Lipid Mediat. 2024. PMID: 38036037 Free PMC article. Review.
-
The applied biochemistry of PEDF and implications for tissue homeostasis.Growth Factors. 2010 Aug;28(4):280-5. doi: 10.3109/08977191003604513. Growth Factors. 2010. PMID: 20166889 Free PMC article. Review.
Cited by
-
Major Maternal Dietary Patterns during Early Pregnancy and Their Association with Neonatal Anthropometric Measurement.Biomed Res Int. 2018 May 31;2018:4692193. doi: 10.1155/2018/4692193. eCollection 2018. Biomed Res Int. 2018. PMID: 29955602 Free PMC article.
-
Corneal Keratocyte Density and Corneal Nerves Are Reduced in Patients With Severe Obesity and Improve After Bariatric Surgery.Invest Ophthalmol Vis Sci. 2021 Jan 4;62(1):20. doi: 10.1167/iovs.62.1.20. Invest Ophthalmol Vis Sci. 2021. PMID: 33475689 Free PMC article.
-
Effects of Different n6/n3 PUFAs Dietary Ratio on Cardiac Diabetic Neuropathy.Nutrients. 2020 Sep 10;12(9):2761. doi: 10.3390/nu12092761. Nutrients. 2020. PMID: 32927766 Free PMC article.
-
Mouse strains and sexual divergence in corneal innervation and nerve regeneration.FASEB J. 2019 Mar;33(3):4598-4609. doi: 10.1096/fj.201801957R. Epub 2018 Dec 18. FASEB J. 2019. PMID: 30561223 Free PMC article.
-
Mouse Models of Inherited Retinal Degeneration with Photoreceptor Cell Loss.Cells. 2020 Apr 10;9(4):931. doi: 10.3390/cells9040931. Cells. 2020. PMID: 32290105 Free PMC article. Review.
References
-
- Müller L. J., Marfurt C. F., Kruse F., and Tervo T. M. (2003) Corneal nerves: structure, contents and function. Exp. Eye Res. 76, 521–542 - PubMed
-
- Chao C., Golebiowski B., and Stapleton F. (2014) The role of corneal innervation in LASIK-induced neuropathic dry eye. Ocul. Surf. 12, 32–45 - PubMed
-
- Erie J. C., McLaren J. W., Hodge D. O., and Bourne W. M. (2005) Recovery of corneal subbasal nerve density after PRK and LASIK. Am. J. Ophthalmol. 140, 1059–1064 - PubMed
-
- Kymionis G. D., Tsiklis N., Pallikaris A. I., Bouzoukis D. I., and Pallikaris I. G. (2007) Fifteen-year follow-up after LASIK: case report. J. Refract. Surg. 23, 937–940 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

