Defining a mechanistic link between pigment epithelium-derived factor, docosahexaenoic acid, and corneal nerve regeneration
- PMID: 28972155
- PMCID: PMC5682960
- DOI: 10.1074/jbc.M117.801472
Defining a mechanistic link between pigment epithelium-derived factor, docosahexaenoic acid, and corneal nerve regeneration
Abstract
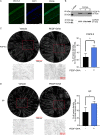
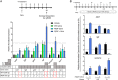
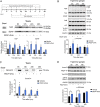
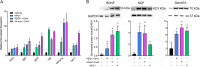
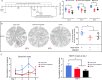
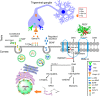
The cornea is densely innervated to sustain the integrity of the ocular surface. Corneal nerve damage produced by aging, diabetes, refractive surgeries, and viral or bacterial infections impairs tear production, the blinking reflex, and epithelial wound healing, resulting in loss of transparency and vision. A combination of the known neuroprotective molecule, pigment epithelium-derived factor (PEDF) plus docosahexaenoic acid (DHA), has been shown to stimulate corneal nerve regeneration, but the mechanisms involved are unclear. Here, we sought to define the molecular events of this effect in an in vivo mouse injury model. We first confirmed that PEDF + DHA increased nerve regeneration in the mouse cornea. Treatment with PEDF activates the phospholipase A2 activity of the PEDF-receptor (PEDF-R) leading to the release of DHA; this free DHA led to enhanced docosanoid synthesis and induction of bdnf, ngf, and the axon growth promoter semaphorin 7a (sema7a), and as a consequence, their products appeared in the mouse tears. Surprisingly, corneal injury and treatment with PEDF + DHA induced transcription of neuropeptide y (npy), small proline-rich protein 1a (sprr1a), and vasoactive intestinal peptide (vip) in the trigeminal ganglia (TG). The PEDF-R inhibitor, atglistatin, blocked all of these changes in the cornea and TG. In conclusion, we uncovered here an active cornea-TG axis, driven by PEDF-R activation, that fosters axon outgrowth in the cornea.
Keywords: DHA; NGF; PEDF; Sema7A; adipose triglyceride lipase; brain-derived neurotrophic factor (BDNF); cornea; lipid signaling; neuropeptides; phospholipase A.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
-
- Müller L. J., Marfurt C. F., Kruse F., and Tervo T. M. (2003) Corneal nerves: structure, contents and function. Exp. Eye Res. 76, 521–542 - PubMed
-
- Chao C., Golebiowski B., and Stapleton F. (2014) The role of corneal innervation in LASIK-induced neuropathic dry eye. Ocul. Surf. 12, 32–45 - PubMed
-
- Erie J. C., McLaren J. W., Hodge D. O., and Bourne W. M. (2005) Recovery of corneal subbasal nerve density after PRK and LASIK. Am. J. Ophthalmol. 140, 1059–1064 - PubMed
-
- Kymionis G. D., Tsiklis N., Pallikaris A. I., Bouzoukis D. I., and Pallikaris I. G. (2007) Fifteen-year follow-up after LASIK: case report. J. Refract. Surg. 23, 937–940 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

