Ligand-dependent corepressor (LCoR) represses the transcription factor C/EBPβ during early adipocyte differentiation
- PMID: 28972158
- PMCID: PMC5704479
- DOI: 10.1074/jbc.M117.793984
Ligand-dependent corepressor (LCoR) represses the transcription factor C/EBPβ during early adipocyte differentiation
Abstract
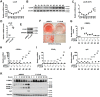
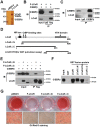
Nuclear receptors (NRs) regulate gene transcription by recruiting coregulators, involved in chromatin remodeling and assembly of the basal transcription machinery. The NR-associated protein ligand-dependent corepressor (LCoR) has previously been shown to suppress hepatic lipogenesis by decreasing the binding of steroid receptor coactivators to thyroid hormone receptor. However, the role of LCoR in adipogenesis has not been established. Here, we show that LCoR expression is reduced in the early stage of adipogenesis in vitro LCoR overexpression inhibited 3T3-L1 adipocyte differentiation, whereas LCoR knockdown promoted it. Using an unbiased affinity purification approach, we identified CCAAT/enhancer-binding protein β (C/EBPβ), a key transcriptional regulator in early adipogenesis, and corepressor C-terminal binding proteins as potential components of an LCoR-containing complex in 3T3-L1 adipocytes. We found that LCoR directly interacts with C/EBPβ through its C-terminal helix-turn-helix domain, required for LCoR's inhibitory effects on adipogenesis. LCoR overexpression also inhibited C/EBPβ transcriptional activity, leading to inhibition of mitotic clonal expansion and transcriptional repression of C/EBPα and peroxisome proliferator-activated receptor γ2 (PPARγ2). However, LCoR overexpression did not affect the recruitment of C/EBPβ to the promoters of C/EBPα and PPARγ2 in 3T3-L1 adipocytes. Of note, restoration of PPARγ2 or C/EBPα expression attenuated the inhibitory effect of LCoR on adipogenesis. Mechanistically, LCoR suppressed C/EBPβ-mediated transcription by recruiting C-terminal binding proteins to the C/EBPα and PPARγ2 promoters and by modulating histone modifications. Taken together, our results indicate that LCoR negatively regulates early adipogenesis by repressing C/EBPβ transcriptional activity and add LCoR to the growing list of transcriptional corepressors of adipogenesis.
Keywords: CCAAT-enhancer-binding protein (C/EBP); adipogenesis; nuclear receptor; peroxisome proliferator-activated receptor (PPAR); transcription corepressor.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures






References
-
- Fowler A. M., and Alarid E. T. (2004) Dynamic control of nuclear receptor transcription. Sci. STKE 2004, pe51. - PubMed
-
- Hermanson O., Glass C. K., and Rosenfeld M. G. (2002) Nuclear receptor coregulators: multiple modes of modification. Trends Endocrinol. Metab. 13, 55–60 - PubMed
-
- Collingwood T. N., Urnov F. D., and Wolffe A. P. (1999) Nuclear receptors: coactivators, corepressors and chromatin remodeling in the control of transcription. J. Mol. Endocrinol. 23, 255–275 - PubMed
-
- Fernandes I., Bastien Y., Wai T., Nygard K., Lin R., Cormier O., Lee H. S., Eng F., Bertos N. R., Pelletier N., Mader S., Han V. K., Yang X. J., and White J. H. (2003) Ligand-dependent nuclear receptor corepressor LCoR functions by histone deacetylase-dependent and -independent mechanisms. Mol. Cell 11, 139–150 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

