An inhibitor of the proteasomal deubiquitinating enzyme USP14 induces tau elimination in cultured neurons
- PMID: 28972160
- PMCID: PMC5702663
- DOI: 10.1074/jbc.M117.815126
An inhibitor of the proteasomal deubiquitinating enzyme USP14 induces tau elimination in cultured neurons
Abstract
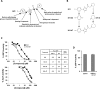
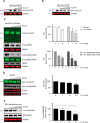
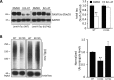
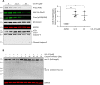
The ubiquitin-proteasome system (UPS) is responsible for most selective protein degradation in eukaryotes and regulates numerous cellular processes, including cell cycle control and protein quality control. A component of this system, the deubiquitinating enzyme USP14, associates with the proteasome where it can rescue substrates from degradation by removal of the ubiquitin tag. We previously found that a small-molecule inhibitor of USP14, known as IU1, can increase the rate of degradation of a subset of proteasome substrates. We report here the synthesis and characterization of 87 variants of IU1, which resulted in the identification of a 10-fold more potent USP14 inhibitor that retains specificity for USP14. The capacity of this compound, IU1-47, to enhance protein degradation in cells was tested using as a reporter the microtubule-associated protein tau, which has been implicated in many neurodegenerative diseases. Using primary neuronal cultures, IU1-47 was found to accelerate the rate of degradation of wild-type tau, the pathological tau mutants P301L and P301S, and the A152T tau variant. We also report that a specific residue in tau, lysine 174, is critical for the IU1-47-mediated tau degradation by the proteasome. Finally, we show that IU1-47 stimulates autophagic flux in primary neurons. In summary, these findings provide a powerful research tool for investigating the complex biology of USP14.
Keywords: IU1; IU1-47; neurodegenerative disease; proteasome; small molecule; tauopathy; ubiquitin.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
Patents 8933087 and 9201073 are held on IU1, IU1-47, and USP14 inhibition. A patent application on this work has been filed by Harvard University on behalf of the authors
Figures



Similar articles
-
Neurotoxic mechanisms by which the USP14 inhibitor IU1 depletes ubiquitinated proteins and Tau in rat cerebral cortical neurons: Relevance to Alzheimer's disease.Biochim Biophys Acta Mol Basis Dis. 2017 Jun;1863(6):1157-1170. doi: 10.1016/j.bbadis.2017.03.017. Epub 2017 Apr 1. Biochim Biophys Acta Mol Basis Dis. 2017. PMID: 28372990 Free PMC article.
-
Ubiquitin-specific protease 14 regulates c-Jun N-terminal kinase signaling at the neuromuscular junction.Mol Neurodegener. 2015 Jan 10;10:3. doi: 10.1186/1750-1326-10-3. Mol Neurodegener. 2015. PMID: 25575639 Free PMC article.
-
The deubiquitinating enzyme Usp14 allosterically inhibits multiple proteasomal activities and ubiquitin-independent proteolysis.J Biol Chem. 2017 Jun 9;292(23):9830-9839. doi: 10.1074/jbc.M116.763128. Epub 2017 Apr 17. J Biol Chem. 2017. PMID: 28416611 Free PMC article.
-
Small-Molecule Inhibitors Targeting Proteasome-Associated Deubiquitinases.Int J Mol Sci. 2021 Jun 9;22(12):6213. doi: 10.3390/ijms22126213. Int J Mol Sci. 2021. PMID: 34207520 Free PMC article. Review.
-
Ubiquitin-specific protease 14 is a new therapeutic target for the treatment of diseases.J Cell Physiol. 2021 May;236(5):3396-3405. doi: 10.1002/jcp.30124. Epub 2020 Nov 1. J Cell Physiol. 2021. PMID: 33135160 Review.
Cited by
-
Ubiquitin-modifying enzymes in Huntington's disease.Front Mol Biosci. 2023 Feb 8;10:1107323. doi: 10.3389/fmolb.2023.1107323. eCollection 2023. Front Mol Biosci. 2023. PMID: 36926679 Free PMC article. Review.
-
Ubiquitin and Ubiquitin-like Proteins in Cancer, Neurodegenerative Disorders, and Heart Diseases.Int J Mol Sci. 2022 May 2;23(9):5053. doi: 10.3390/ijms23095053. Int J Mol Sci. 2022. PMID: 35563444 Free PMC article. Review.
-
Targeting ubiquitin specific proteases (USPs) in cancer immunotherapy: from basic research to preclinical application.J Exp Clin Cancer Res. 2023 Sep 1;42(1):225. doi: 10.1186/s13046-023-02805-y. J Exp Clin Cancer Res. 2023. PMID: 37658402 Free PMC article. Review.
-
Proteostasis in Huntington's disease: disease mechanisms and therapeutic opportunities.Acta Pharmacol Sin. 2018 May;39(5):754-769. doi: 10.1038/aps.2018.11. Epub 2018 Apr 5. Acta Pharmacol Sin. 2018. PMID: 29620053 Free PMC article. Review.
-
Mechanisms and regulation of substrate degradation by the 26S proteasome.Nat Rev Mol Cell Biol. 2025 Feb;26(2):104-122. doi: 10.1038/s41580-024-00778-0. Epub 2024 Oct 3. Nat Rev Mol Cell Biol. 2025. PMID: 39362999 Review.
References
-
- Goldberg A. L. (2007) Functions of the proteasome: from protein degradation and immune surveillance to cancer therapy. Biochem. Soc. Trans. 35, 12–17 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

