Isomer activation controls stereospecificity of class I fructose-1,6-bisphosphate aldolases
- PMID: 28972169
- PMCID: PMC5712624
- DOI: 10.1074/jbc.M117.811034
Isomer activation controls stereospecificity of class I fructose-1,6-bisphosphate aldolases
Abstract
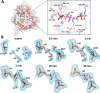
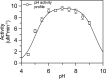
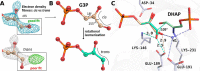
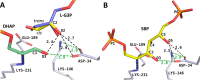
Fructose-1,6-bisphosphate (FBP) aldolase, a glycolytic enzyme, catalyzes the reversible and stereospecific aldol addition of dihydroxyacetone phosphate (DHAP) and d-glyceraldehyde 3-phosphate (d-G3P) by an unresolved mechanism. To afford insight into the molecular determinants of FBP aldolase stereospecificity during aldol addition, a key ternary complex formed by DHAP and d-G3P, comprising 2% of the equilibrium population at physiological pH, was cryotrapped in the active site of Toxoplasma gondii aldolase crystals to high resolution. The growth of T. gondii aldolase crystals in acidic conditions enabled trapping of the ternary complex as a dominant population. The obligate 3(S)-4(R) stereochemistry at the nascent C3-C4 bond of FBP requires a si-face attack by the covalent DHAP nucleophile on the d-G3P aldehyde si-face in the active site. The cis-isomer of the d-G3P aldehyde, representing the dominant population trapped in the ternary complex, would lead to re-face attack on the aldehyde and yield tagatose 1,6-bisphosphate, a competitive inhibitor of the enzyme. We propose that unhindered rotational isomerization by the d-G3P aldehyde moiety in the ternary complex generates the active trans-isomer competent for carbonyl bond activation by active-site residues, thereby enabling si-face attack by the DHAP enamine. C-C bond formation by the cis-isomer is suppressed by hydrogen bonding of the cis-aldehyde carbonyl with the DHAP enamine phosphate dianion through a tetrahedrally coordinated water molecule. The active site geometry further suppresses C-C bond formation with the l-G3P enantiomer of d-G3P. Understanding C-C formation is of fundamental importance in biological reactions and has considerable relevance to biosynthetic reactions in organic chemistry.
Keywords: crystal structure; enzyme catalysis; enzyme mechanism; glycolysis; stereoselectivity.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
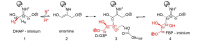
Similar articles
-
Structure of a class I tagatose-1,6-bisphosphate aldolase: investigation into an apparent loss of stereospecificity.J Biol Chem. 2010 Jul 2;285(27):21143-52. doi: 10.1074/jbc.M109.080358. Epub 2010 Apr 28. J Biol Chem. 2010. PMID: 20427286 Free PMC article.
-
Structural insights into the substrate binding and stereoselectivity of giardia fructose-1,6-bisphosphate aldolase.Biochemistry. 2009 Apr 14;48(14):3186-96. doi: 10.1021/bi9001166. Biochemistry. 2009. PMID: 19236002 Free PMC article.
-
Active site remodeling during the catalytic cycle in metal-dependent fructose-1,6-bisphosphate aldolases.J Biol Chem. 2018 May 18;293(20):7737-7753. doi: 10.1074/jbc.RA117.001098. Epub 2018 Mar 28. J Biol Chem. 2018. PMID: 29593097 Free PMC article.
-
Sweet siblings with different faces: the mechanisms of FBP and F6P aldolase, transaldolase, transketolase and phosphoketolase revisited in light of recent structural data.Bioorg Chem. 2014 Dec;57:263-280. doi: 10.1016/j.bioorg.2014.09.001. Epub 2014 Sep 16. Bioorg Chem. 2014. PMID: 25267444 Review.
-
Recent advances in the synthesis of rare sugars using DHAP-dependent aldolases.Carbohydr Res. 2017 Nov 27;452:108-115. doi: 10.1016/j.carres.2017.10.009. Epub 2017 Oct 18. Carbohydr Res. 2017. PMID: 29096183 Review.
Cited by
-
Structural and Functional Characterization of YdjI, an Aldolase of Unknown Specificity in Escherichia coli K12.Biochemistry. 2019 Aug 6;58(31):3340-3353. doi: 10.1021/acs.biochem.9b00326. Epub 2019 Jul 26. Biochemistry. 2019. PMID: 31322866 Free PMC article.
-
Cellular and Molecular Targets of Nucleotide-Tagged Trithiolato-Bridged Arene Ruthenium Complexes in the Protozoan Parasites Toxoplasma gondii and Trypanosoma brucei.Int J Mol Sci. 2021 Oct 5;22(19):10787. doi: 10.3390/ijms221910787. Int J Mol Sci. 2021. PMID: 34639127 Free PMC article.
References
-
- Grazi E., Rowley P. T., Cheng T., Tchola O., and Horecker B. L. (1962) The mechanism of action of aldolases. III. Schiff base formation with lysine. Biochem. Biophys. Res. Commun. 9, 38–43 - PubMed
-
- St-Jean M., Lafrance-Vanasse J., Liotard B., and Sygusch J. (2005) High resolution reaction intermediates of rabbit muscle fructose-1,6-bisphosphate aldolase: substrate cleavage and induced fit. J. Biol. Chem. 280, 27262–27270 - PubMed
-
- St-Jean M., and Sygusch J. (2007) Stereospecific proton transfer by a mobile catalyst in mammalian fructose-1,6-bisphosphate aldolase. J. Biol. Chem. 282, 31028–31037 - PubMed
-
- Rose I. A., and O'Connell E. L. (1969) Studies on the interaction of aldolase with substrate analogues. J. Biol. Chem. 244, 126–134 - PubMed
-
- Hartman F. C., and Barker R. (1965) An exploration of the active site of aldolase using structural analogs of fructose diphosphate. Biochemistry 4, 1068–1075 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

