Inhibition of the sarco/endoplasmic reticulum (ER) Ca2+-ATPase by thapsigargin analogs induces cell death via ER Ca2+ depletion and the unfolded protein response
- PMID: 28972171
- PMCID: PMC5712609
- DOI: 10.1074/jbc.M117.796920
Inhibition of the sarco/endoplasmic reticulum (ER) Ca2+-ATPase by thapsigargin analogs induces cell death via ER Ca2+ depletion and the unfolded protein response
Abstract
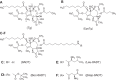
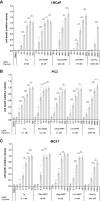
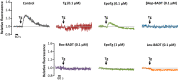
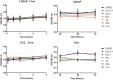
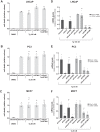
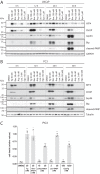
Calcium (Ca2+) is a fundamental regulator of cell signaling and function. Thapsigargin (Tg) blocks the sarco/endoplasmic reticulum (ER) Ca2+-ATPase (SERCA), disrupts Ca2+ homeostasis, and causes cell death. However, the exact mechanisms whereby SERCA inhibition induces cell death are incompletely understood. Here, we report that low (0.1 μm) concentrations of Tg and Tg analogs with various long-chain substitutions at the O-8 position extensively inhibit SERCA1a-mediated Ca2+ transport. We also found that, in both prostate and breast cancer cells, exposure to Tg or Tg analogs for 1 day caused extensive drainage of the ER Ca2+ stores. This Ca2+ depletion was followed by markedly reduced cell proliferation rates and morphological changes that developed over 2-4 days and culminated in cell death. Interestingly, these changes were not accompanied by bulk increases in cytosolic Ca2+ levels. Moreover, knockdown of two key store-operated Ca2+ entry (SOCE) components, Orai1 and STIM1, did not reduce Tg cytotoxicity, indicating that SOCE and Ca2+ entry are not critical for Tg-induced cell death. However, we observed a correlation between the abilities of Tg and Tg analogs to deplete ER Ca2+ stores and their detrimental effects on cell viability. Furthermore, caspase activation and cell death were associated with a sustained unfolded protein response. We conclude that ER Ca2+ drainage and sustained unfolded protein response activation are key for initiation of apoptosis at low concentrations of Tg and Tg analogs, whereas high cytosolic Ca2+ levels and SOCE are not required.
Keywords: apoptosis; calcium; endoplasmic reticulum (ER); stromal interaction molecule 1 (STIM1); unfolded protein response (UPR).
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

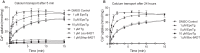

Similar articles
-
Nonlinear relationship between ER Ca2+ depletion versus induction of the unfolded protein response, autophagy inhibition, and cell death.Cell Calcium. 2018 Dec;76:48-61. doi: 10.1016/j.ceca.2018.09.005. Epub 2018 Sep 17. Cell Calcium. 2018. PMID: 30261424
-
Paradoxical effects of sarco/endoplasmic reticulum Ca(2+)-ATPase (SERCA) activator gingerol on NG115-401L neuronal cells: failure to augment ER Ca(2+) uptake and protect against ER stress-induced cell death.Eur J Pharmacol. 2015 Sep 5;762:165-73. doi: 10.1016/j.ejphar.2015.05.055. Epub 2015 May 29. Eur J Pharmacol. 2015. PMID: 26033206
-
Differential effects of thapsigargin analogues on apoptosis of prostate cancer cells: complex regulation by intracellular calcium.FEBS J. 2013 Nov;280(21):5430-40. doi: 10.1111/febs.12475. Epub 2013 Sep 2. FEBS J. 2013. PMID: 23927406
-
Privileged coupling between Ca(2+) entry through plasma membrane store-operated Ca(2+) channels and the endoplasmic reticulum Ca(2+) pump.Mol Cell Endocrinol. 2012 Apr 28;353(1-2):37-44. doi: 10.1016/j.mce.2011.08.021. Epub 2011 Aug 24. Mol Cell Endocrinol. 2012. PMID: 21878366 Review.
-
Key components of store-operated Ca2+ entry in non-excitable cells.J Pharmacol Sci. 2014;125(4):340-6. doi: 10.1254/jphs.14r06cp. Epub 2014 Jul 17. J Pharmacol Sci. 2014. PMID: 25030742 Review.
Cited by
-
STING, the Endoplasmic Reticulum, and Mitochondria: Is Three a Crowd or a Conversation?Front Immunol. 2021 Jan 21;11:611347. doi: 10.3389/fimmu.2020.611347. eCollection 2020. Front Immunol. 2021. PMID: 33552072 Free PMC article. Review.
-
Thapsigargin-From Traditional Medicine to Anticancer Drug.Int J Mol Sci. 2020 Dec 22;22(1):4. doi: 10.3390/ijms22010004. Int J Mol Sci. 2020. PMID: 33374919 Free PMC article. Review.
-
NREP contributes to development of NAFLD by regulating one-carbon metabolism in primary human hepatocytes.Cell Chem Biol. 2023 Sep 21;30(9):1144-1155.e4. doi: 10.1016/j.chembiol.2023.06.001. Epub 2023 Jun 23. Cell Chem Biol. 2023. PMID: 37354909 Free PMC article.
-
Synergistic antitumor effect of a penicillin derivative combined with thapsigargin in melanoma cells.J Cancer Res Clin Oncol. 2022 Dec;148(12):3361-3373. doi: 10.1007/s00432-022-04129-4. Epub 2022 Jun 25. J Cancer Res Clin Oncol. 2022. PMID: 35751681 Free PMC article.
-
Thapsigargin-Stimulated LAD2 Human Mast Cell Line Is a Potent Cellular Adjuvant for the Maturation of Monocyte-Derived Dendritic Cells for Adoptive Cellular Immunotherapy.Int J Mol Sci. 2021 Apr 12;22(8):3978. doi: 10.3390/ijms22083978. Int J Mol Sci. 2021. PMID: 33921475 Free PMC article.
References
-
- Wang M., and Kaufman R. J. (2014) The impact of the endoplasmic reticulum protein-folding environment on cancer development. Nat. Rev. Cancer 14, 581–597 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

