Post-translational modifications clustering within proteolytic domains decrease mutant huntingtin toxicity
- PMID: 28972180
- PMCID: PMC5702665
- DOI: 10.1074/jbc.M117.782300
Post-translational modifications clustering within proteolytic domains decrease mutant huntingtin toxicity
Abstract
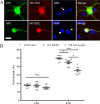
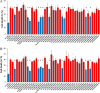
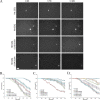
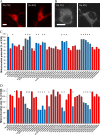
Huntington's disease (HD) is caused in large part by a polyglutamine expansion within the huntingtin (Htt) protein. Post-translational modifications (PTMs) control and regulate many protein functions and cellular pathways, and PTMs of mutant Htt are likely important modulators of HD pathogenesis. Alterations of selected numbers of PTMs of Htt fragments have been shown to modulate Htt cellular localization and toxicity. In this study, we systematically introduced site-directed alterations in individual phosphorylation and acetylation sites in full-length Htt constructs. The effects of each of these PTM alteration constructs were tested on cell toxicity using our nuclear condensation assay and on mitochondrial viability by measuring mitochondrial potential and size. Using these functional assays in primary neurons, we identified several PTMs whose alteration can block neuronal toxicity and prevent potential loss and swelling of the mitochondria caused by mutant Htt. These PTMs included previously described sites such as serine 116 and newly found sites such as serine 2652 throughout the protein. We found that these functionally relevant sites are clustered in protease-sensitive domains throughout full-length Htt. These findings advance our understanding of the Htt PTM code and its role in HD pathogenesis. Because PTMs are catalyzed by enzymes, the toxicity-modulating Htt PTMs identified here may be promising therapeutic targets for managing HD.
Keywords: Huntington disease; neurodegeneration; neuron; phosphorylation; toxicity.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures
References
-
- The Huntington's Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington's disease chromosomes. Cell 72, 971–983 - PubMed
-
- Xu Y., Deng Y., and Qing H. (2015) The phosphorylation of α-synuclein: development and implication for the mechanism and therapy of the Parkinson's disease. J. Neurochem. 135, 4–18 - PubMed
-
- Hasegawa M., Nonaka T., Tsuji H., Tamaoka A., Yamashita M., Kametani F., Yoshida M., Arai T., and Akiyama H. (2011) Molecular dissection of tdp-43 proteinopathies. J. Mol. Neurosci. 45, 480–485 - PubMed
-
- Aronin N., and DiFiglia M. (2014) Huntingtin-lowering strategies in Huntington's disease: antisense oligonucleotides, small RNAs, and gene editing. Mov. Disord 29, 1455–1461 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

