Urokinase-type plasminogen activator (uPA) is critical for progression of tuberous sclerosis complex 2 (TSC2)-deficient tumors
- PMID: 28972182
- PMCID: PMC5733590
- DOI: 10.1074/jbc.M117.799593
Urokinase-type plasminogen activator (uPA) is critical for progression of tuberous sclerosis complex 2 (TSC2)-deficient tumors
Abstract
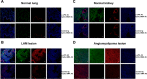
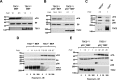
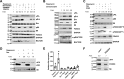
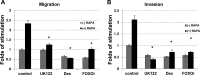
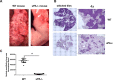
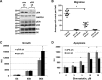
Lymphangioleiomyomatosis (LAM) is a fatal lung disease associated with germline or somatic inactivating mutations in tuberous sclerosis complex genes (TSC1 or TSC2). LAM is characterized by neoplastic growth of smooth muscle-α-actin-positive cells that destroy lung parenchyma and by the formation of benign renal neoplasms called angiolipomas. The mammalian target of rapamycin complex 1 (mTORC1) inhibitor rapamycin slows progression of these diseases but is not curative and associated with notable toxicity at clinically effective doses, highlighting the need for better understanding LAM's molecular etiology. We report here that LAM lesions and angiomyolipomas overexpress urokinase-type plasminogen activator (uPA). Tsc1-/- and Tsc2-/- mouse embryonic fibroblasts expressed higher uPA levels than their WT counterparts, resulting from the TSC inactivation. Inhibition of uPA expression in Tsc2-null cells reduced the growth and invasiveness and increased susceptibility to apoptosis. However, rapamycin further increased uPA expression in TSC2-null tumor cells and immortalized TSC2-null angiomyolipoma cells, but not in cells with intact TSC. Induction of glucocorticoid receptor signaling or forkhead box (FOXO) 1/3 inhibition abolished the rapamycin-induced uPA expression in TSC-compromised cells. Moreover, rapamycin-enhanced migration of TSC2-null cells was inhibited by the uPA inhibitor UK122, dexamethasone, and a FOXO inhibitor. uPA-knock-out mice developed fewer and smaller TSC2-null lung tumors, and introduction of uPA shRNA in tumor cells or amiloride-induced uPA inhibition reduced tumorigenesis in vivo These findings suggest that interference with the uPA-dependent pathway, when used along with rapamycin, might attenuate LAM progression and potentially other TSC-related disorders.
Keywords: TOR complex (TORC); lung; mTOR complex (mTORC); mammalian target of rapamycin (mTOR); plasminogen; tuberous sclerosis complex (TSC); tumor cell biology; urokinase receptor.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


Similar articles
-
Rapamycin-insensitive up-regulation of adipocyte phospholipase A2 in tuberous sclerosis and lymphangioleiomyomatosis.PLoS One. 2014 Oct 27;9(10):e104809. doi: 10.1371/journal.pone.0104809. eCollection 2014. PLoS One. 2014. PMID: 25347447 Free PMC article.
-
Rapamycin-insensitive up-regulation of MMP2 and other genes in tuberous sclerosis complex 2-deficient lymphangioleiomyomatosis-like cells.Am J Respir Cell Mol Biol. 2010 Feb;42(2):227-34. doi: 10.1165/rcmb.2009-0050OC. Epub 2009 Apr 24. Am J Respir Cell Mol Biol. 2010. PMID: 19395678 Free PMC article.
-
Whole Exome Sequencing Identifies TSC1/TSC2 Biallelic Loss as the Primary and Sufficient Driver Event for Renal Angiomyolipoma Development.PLoS Genet. 2016 Aug 5;12(8):e1006242. doi: 10.1371/journal.pgen.1006242. eCollection 2016 Aug. PLoS Genet. 2016. PMID: 27494029 Free PMC article.
-
Mammalian target of rapamycin signaling and autophagy: roles in lymphangioleiomyomatosis therapy.Proc Am Thorac Soc. 2010 Feb;7(1):48-53. doi: 10.1513/pats.200909-104JS. Proc Am Thorac Soc. 2010. PMID: 20160148 Free PMC article. Review.
-
Clinical and molecular insights into lymphangioleiomyomatosis.Sarcoidosis Vasc Diffuse Lung Dis. 2005 Dec;22 Suppl 1:S49-66. Sarcoidosis Vasc Diffuse Lung Dis. 2005. PMID: 16457017 Review.
Cited by
-
Screening of 5- and 6-Substituted Amiloride Libraries Identifies Dual-uPA/NHE1 Active and Single Target-Selective Inhibitors.Int J Mol Sci. 2021 Mar 15;22(6):2999. doi: 10.3390/ijms22062999. Int J Mol Sci. 2021. PMID: 33804289 Free PMC article.
-
SIRT1 inhibits chemoresistance and cancer stemness of gastric cancer by initiating an AMPK/FOXO3 positive feedback loop.Cell Death Dis. 2020 Feb 12;11(2):115. doi: 10.1038/s41419-020-2308-4. Cell Death Dis. 2020. PMID: 32051395 Free PMC article.
-
Nischarin Deletion Reduces Oxidative Metabolism and Overall ATP: A Study Using a Novel NISCHΔ5-6 Knockout Mouse Model.Int J Mol Sci. 2022 Jan 25;23(3):1374. doi: 10.3390/ijms23031374. Int J Mol Sci. 2022. PMID: 35163298 Free PMC article.
-
The epithelial sodium channel has a role in breast cancer cell proliferation.Breast Cancer Res Treat. 2021 May;187(1):31-43. doi: 10.1007/s10549-021-06133-7. Epub 2021 Feb 25. Breast Cancer Res Treat. 2021. PMID: 33630195
-
Possible Novel Therapeutic Targets in Lymphangioleiomyomatosis Treatment.Front Med (Lausanne). 2020 Sep 24;7:554134. doi: 10.3389/fmed.2020.554134. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33072782 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

