A unique cysteine-rich zinc finger domain present in a majority of class II ribonucleotide reductases mediates catalytic turnover
- PMID: 28972190
- PMCID: PMC5704485
- DOI: 10.1074/jbc.M117.806331
A unique cysteine-rich zinc finger domain present in a majority of class II ribonucleotide reductases mediates catalytic turnover
Abstract
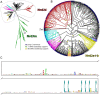
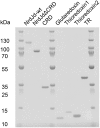
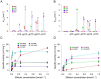
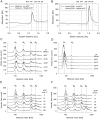
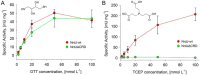
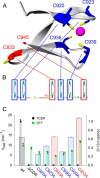
Ribonucleotide reductases (RNRs) catalyze the reduction of ribonucleotides to the corresponding deoxyribonucleotides, used in DNA synthesis and repair. Two different mechanisms help deliver the required electrons to the RNR active site. Formate can be used as reductant directly in the active site, or glutaredoxins or thioredoxins reduce a C-terminal cysteine pair, which then delivers the electrons to the active site. Here, we characterized a novel cysteine-rich C-terminal domain (CRD), which is present in most class II RNRs found in microbes. The NrdJd-type RNR from the bacterium Stackebrandtia nassauensis was used as a model enzyme. We show that the CRD is involved in both higher oligomeric state formation and electron transfer to the active site. The CRD-dependent formation of high oligomers, such as tetramers and hexamers, was induced by addition of dATP or dGTP, but not of dTTP or dCTP. The electron transfer was mediated by an array of six cysteine residues at the very C-terminal end, which also coordinated a zinc atom. The electron transfer can also occur between subunits, depending on the enzyme's oligomeric state. An investigation of the native reductant of the system revealed no interaction with glutaredoxins or thioredoxins, indicating that this class II RNR uses a different electron source. Our results indicate that the CRD has a crucial role in catalytic turnover and a potentially new terminal reduction mechanism and suggest that the CRD is important for the activities of many class II RNRs.
Keywords: metal ion–protein interaction; oligomerization; oxidation-reduction (redox); phylogenetics; ribonucleotide reductase; thioredoxin.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


Similar articles
-
A glutaredoxin domain fused to the radical-generating subunit of ribonucleotide reductase (RNR) functions as an efficient RNR reductant.J Biol Chem. 2018 Oct 12;293(41):15889-15900. doi: 10.1074/jbc.RA118.004991. Epub 2018 Aug 30. J Biol Chem. 2018. PMID: 30166338 Free PMC article.
-
Diversity in Overall Activity Regulation of Ribonucleotide Reductase.J Biol Chem. 2015 Jul 10;290(28):17339-48. doi: 10.1074/jbc.M115.649624. Epub 2015 May 13. J Biol Chem. 2015. PMID: 25971975 Free PMC article.
-
Phosphines are ribonucleotide reductase reductants that act via C-terminal cysteines similar to thioredoxins and glutaredoxins.Sci Rep. 2014 Jul 2;4:5539. doi: 10.1038/srep05539. Sci Rep. 2014. PMID: 24986213 Free PMC article.
-
Structure, function, and mechanism of ribonucleotide reductases.Biochim Biophys Acta. 2004 Jun 1;1699(1-2):1-34. doi: 10.1016/j.bbapap.2004.02.007. Biochim Biophys Acta. 2004. PMID: 15158709 Review.
-
Structure and function of the radical enzyme ribonucleotide reductase.Prog Biophys Mol Biol. 2001 Nov;77(3):177-268. doi: 10.1016/s0079-6107(01)00014-1. Prog Biophys Mol Biol. 2001. PMID: 11796141 Review.
Cited by
-
Analysis of insertions and extensions in the functional evolution of the ribonucleotide reductase family.Protein Sci. 2022 Dec;31(12):e4483. doi: 10.1002/pro.4483. Protein Sci. 2022. PMID: 36307939 Free PMC article.
-
Structural determinants and distribution of phosphate specificity in ribonucleotide reductases.J Biol Chem. 2021 Aug;297(2):101008. doi: 10.1016/j.jbc.2021.101008. Epub 2021 Jul 24. J Biol Chem. 2021. PMID: 34314684 Free PMC article.
-
Foam fractionation Tags (F-Tags) enabling surfactant-free, activity-preserving recovery of enzymes.Appl Microbiol Biotechnol. 2024 Jan 17;108(1):140. doi: 10.1007/s00253-023-12837-1. Appl Microbiol Biotechnol. 2024. PMID: 38231394 Free PMC article.
-
Metal-free ribonucleotide reduction powered by a DOPA radical in Mycoplasma pathogens.Nature. 2018 Nov;563(7731):416-420. doi: 10.1038/s41586-018-0653-6. Epub 2018 Oct 31. Nature. 2018. PMID: 30429545 Free PMC article.
-
A Novel One-Pot Enzyme Cascade for the Biosynthesis of Cladribine Triphosphate.Biomolecules. 2021 Feb 25;11(3):346. doi: 10.3390/biom11030346. Biomolecules. 2021. PMID: 33668847 Free PMC article.
References
-
- Nordlund P., and Reichard P. (2006) Ribonucleotide reductases. Annu. Rev. Biochem. 75, 681–706 - PubMed
-
- Stubbe J., Nocera D. G., Yee C. S., and Chang M. C. Y. (2003) Radical initiation in the class I ribonucleotide reductase: long-range proton-coupled electron transfer. Chem. Rev. 103, 2167–2201 - PubMed
-
- Åberg A, Hahne S., Karlsson M., Larsson Å., Ormö M., Åhgren A., and Sjöberg B. M. (1989) Evidence for two different classes of redox-active cysteines in ribonucleotide reductase of Escherichia coli. J. Biol. Chem. 264, 12249–12252 - PubMed
-
- Mao S. S., Yu G. X., Chalfoun D., and Stubbe J. (1992) Characterization of C439SR1, a mutant of Escherichia coli ribonucleotide diphosphate reductase: Evidence that C439 is a residue essential for nucleotide reduction and C439SR1 is a protein possessing novel thioredoxin-like activity. Biochemistry 31, 9752–9759 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

