Shared decision-making for people with asthma
- PMID: 28972652
- PMCID: PMC6485676
- DOI: 10.1002/14651858.CD012330.pub2
Shared decision-making for people with asthma
Abstract
Background: Asthma is a chronic inflammatory disease that affects the airways and is common in both adults and children. It is characterised by symptoms including wheeze, shortness of breath, chest tightness, and cough. People with asthma may be helped to manage their condition through shared decision-making (SDM). SDM involves at least two participants (the medical practitioner and the patient) and mutual sharing of information, including the patient's values and preferences, to build consensus about favoured treatment that culminates in an agreed action. Effective self-management is particularly important for people with asthma, and SDM may improve clinical outcomes and quality of life by educating patients and empowering them to be actively involved in their own health.
Objectives: To assess benefits and potential harms of shared decision-making for adults and children with asthma.
Search methods: We searched the Cochrane Airways Trials Register, which contains studies identified in several sources including CENTRAL, MEDLINE, and Embase. We also searched clinical trials registries and checked the reference lists of included studies. We conducted the most recent searches on 29 November 2016.
Selection criteria: We included studies of individual or cluster parallel randomised controlled design conducted to compare an SDM intervention for adults and children with asthma versus a control intervention. We included studies available as full-text reports, those published as abstracts only, and unpublished data, and we placed no restrictions on place, date, or language of publication. We included interventions targeting healthcare professionals or patients, their families or care-givers, or both. We included studies that compared the intervention versus usual care or a minimal control intervention, and those that compared an SDM intervention against another active intervention. We excluded studies of interventions that involved multiple components other than the SDM intervention unless the control group also received these interventions.
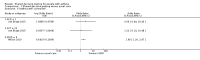
Data collection and analysis: Two review authors independently screened searches, extracted data from included studies, and assessed risk of bias. Primary outcomes were asthma-related quality of life, patient/parent satisfaction, and medication adherence. Secondary outcomes included exacerbations of asthma, asthma control, acceptability/feasibility from the perspective of healthcare professionals, and all adverse events. We graded and presented evidence in a 'Summary of findings' table.We were unable to pool any of the extracted outcome data owing to clinical and methodological heterogeneity but presented findings in forest plots when possible. We narratively described skewed data.
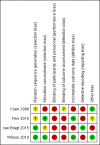
Main results: We included four studies that compared SDM versus control and included a total of 1342 participants. Three studies recruited children with asthma and their care-givers, and one recruited adults with asthma. Three studies took place in the United States, and one in the Netherlands. Trial duration was between 6 and 24 months. One trial delivered the SDM intervention to the medical practitioner, and three trials delivered the SDM intervention directly to the participant. Two paediatric studies involved use of an online portal, followed by face-to-face consultations. One study delivered an SDM intervention or a clinical decision-making intervention through a mixture of face-to-face consultations and telephone calls. The final study randomised paediatric general practice physicians to receive a seminar programme promoting application of SDM principles. All trials were open-label, although one study, which delivered the intervention to physicians, stated that participants were unaware of their physicians' involvement in the trial. We had concerns about selection and attrition bias and selective reporting, and we noted that one study substantially under-recruited participants. The four included studies used different approaches to measure fidelity/intervention adherence and to report study findings.One study involving adults with poorly controlled asthma reported improved quality of life (QOL) for the SDM group compared with the control group, using the Asthma Quality of Life Questionnaire (AQLQ) for assessment (mean difference (MD) 1.90, 95% confidence interval (CI) 1.24 to 2.91), but two other trials did not identify a benefit. Patient/parent satisfaction with the performance of paediatricians was greater in the SDM group in one trial involving children. Medication adherence was better in the SDM group in two studies - one involving adults and one involving children (all medication adherence: MD 0.21, 95% CI 0.11 to 0.31; mean number of controlled medication prescriptions over 26 weeks: 1.1 in the SDM group (n = 26) and 0.7 in the control group (n = 27)). In one study, asthma-related visit rates were lower in the SDM group than in the usual care group (1.0/y vs 1.4/y; P = 0.016), but two other studies did not report a difference in exacerbations nor in prescriptions for short courses of oral steroids. Finally, one study described better odds of reporting no asthma problems in the SDM group than in the usual care group (odds ratio (OR) 1.90, 95% CI 1.26 to 2.87), although two other studies reporting asthma control did not identify a benefit with SDM. We found no information about acceptability of the intervention to the healthcare professional and no information on adverse events. Overall, our confidence in study results ranged from very low to moderate, and we downgraded outcomes owing to risk of bias, imprecision, and indirectness.
Authors' conclusions: Substantial differences between the four included randomised controlled trials (RCTs) indicate that we cannot provide meaningful overall conclusions. Individual studies demonstrated some benefits of SDM over control, in terms of quality of life; patient and parent satisfaction; adherence to prescribed medication; reduction in asthma-related healthcare visits; and improved asthma control. Our confidence in the findings of these individual studies ranges from moderate to very low, and it is important to note that studies did not measure or report adverse events.Future trials should be adequately powered and of sufficient duration to detect differences in patient-important outcomes such as exacerbations and hospitalisations. Use of core asthma outcomes and validated scales when possible would facilitate future meta-analysis. Studies conducted in lower-income settings and including an economic evaluation would be of interest. Investigators should systematically record adverse events, even if none are anticipated. Studies identified to date have not included adolescents; future trials should consider their inclusion. Measuring and reporting of intervention fidelity is also recommended.
Conflict of interest statement
KK is funded to prepare Cochrane reviews by a Programme Grant awarded by the NIHR to the Cochrane Airways Group.
PM has reported no conflicts.
KA is a consultant respiratory paediatrician with respiratory interest in the NHS. He has no alternative sources of funding.
RN is a qualified general practitioner and the deputy Co‐ordinating Editor of Cochrane Airways. She is funded by an NIHR grant to Cochrane Airways.
Figures
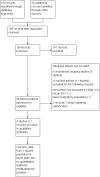





Update of
References
References to studies included in this review
Clark 1998 {published data only}
-
- Clark NM, Gong M, Schork MA, Evans D, Roloff D, Hurwitz M, et al. Impact of education for physicians on patient outcomes. Pediatrics 1998;101(5):831‐6. [CENTRAL: 688433; CRS: 4900100000023539; PUBMED: 9565410] - PubMed
-
- Clark NM, Gong M, Schork MA, Kaciroti N, Evans D, Roloff D, et al. Long‐term effects of asthma education for physicians on patient satisfaction and use of health services. European Respiratory Journal 2000;16(1):15‐21. [CENTRAL: 316303; CRS: 4900100000009731; 4900100000009731; PUBMED: 10933079] - PubMed
-
- Frasca MA. Participation in an interactive seminar improved paediatricians' patient teaching and communication skills: commentary. Evidence‐Based Medicine 1999;4(1):31. [CENTRAL: 310793; CRS: 4900100000009226]
Fiks 2015 {published data only}
-
- NCT01715389. Evaluation of a shared decision making portal for pediatric asthma. clinicaltrials.gov/show/NCT01715389 (first received 10 October 2012). [CRS: 4900132000030028]
van Bragt 2015 {published data only}
-
- NCT01109745. Effectiveness of the pelican Instrument in medical care (PELICANII). clinicaltrials.gov/show/NCT01109745 (first received 22 April 2010).
-
- Bragt S, Bemt L, Kievits R, Merkus P, Weel C, Schermer T. PELICAN: a randomized controlled trial in Dutch General Practices to assess the effectiveness of individualised self‐management for paediatric asthma [Abstract]. 7th International Primary Care Respiratory Group (IPCRG) World Conference; 2014 May 21‐24; Athens. 2014:OR‐009. [CENTRAL: 994138; CRS: 4900126000016465]
-
- Bragt S, Bemt L, Cretier R, Weel C, Merkus P, Schermer T. PELICAN: content evaluation of patient‐centered care for children with asthma based on an online tool. Pediatric Pulmonology 2016;51(10):993‐1003. [CENTRAL: 1152866; CRS: 4900132000019804; PUBMED: 27128738] - PubMed
-
- Bragt S, Bemt L, Kievits R, Merkus P, Weel C, Schermer T. PELICAN: a cluster‐randomized controlled trial in Dutch general practices to assess a self‐management support intervention based on individual goals for children with asthma. Journal of Asthma 2015;52(2):211‐9. [CENTRAL: 1077131; CRS: 4900132000004118; EMBASE: 2015136467; PUBMED: 25166455] - PubMed
-
- Bragt S, Bemt L, Thoonen B, Weel C, Merkus P, Schermer T. PELICAN: a quality of life instrument for childhood asthma: study protocol of two randomized controlled trials in primary and specialized care in the Netherlands. BMC Pediatrics 2012;12(137):137. [CENTRAL: 834422; CRS: 4900100000061225; EMBASE: 2012715722; PUBMED: 22935133] - PMC - PubMed
Wilson 2010 {published data only}
-
- Does shared decision‐making improve asthma outcomes?. https://clinicaltrials.gov/ct2/show/NCT00149526 (accessed 9 September 2016).
-
- NCT00149526. Does shared decision‐making improve asthma outcomes?. https://clinicaltrials.gov/ct2/show/NCT00149526 (first received 6 September 2005). [CRS: 4900100000021091]
-
- NCT00217945. Does shared decision‐making improve adherence in asthma. clinicaltrials.gov/ct2/show/NCT00217945 (first received 19 September 2005).
-
- Wilson SR, Knowles S, Qian Y, Buist AS, Strub P, Lapidus J, et al. Does involving patients in treatment decisions reduce use of asthma rescue medication? [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco. 2007:[A242]. [CENTRAL: 651696; CRS: 4900100000022587]
-
- Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, et al. Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. American Journal of Respiratory and Critical Care Medicine 2010;181(6):566‐77. [CENTRAL: 728584; CRS: 4900100000024367; EMBASE: 2010157400; PUBMED: 20019345] - PMC - PubMed
References to studies excluded from this review
Early 2015 {published data only}
-
- Early F, Everden AJ, O'Brien CM, Fagan PL, Fuld JP. Patient agenda setting in respiratory outpatients: a randomized controlled trial. Chronic Respiratory Disease 2015;12(4):347‐56. [CENTRAL: 1101025; CRS: 4900132000009524; EMBASE: 2015440958; PUBMED: 26272499] - PubMed
Ford 1996 {published data only}
-
- Ford ME, Edwards G, Rodriguez JL, Gibson RC, Tilley BC. An empowerment‐centered, church‐based asthma education program for African American adults. Health and Social Work 1996;21(1):70‐5. - PubMed
Gorelick 2006 {published data only}
-
- Gorelick MH, Meurer JR, Walsh‐Kelly CM, Brousseau DC, Grabowski L, Cohn J, et al. Emergency department allies: a controlled trial of two emergency department‐based follow‐up interventions to improve asthma outcomes in children. Pediatrics 2006;117(4 Suppl 2):S127‐34. - PubMed
Moffat 2008 {published data only}
-
- Cleland A, Price DB, Hall S. Implementing asthma action plans: practice nurse training in patient centered communication skills has no impact on patient outcomes. Thorax 2004;59:ii32.
-
- Cleland JA, Price DB, Moffat M, Hall S. Training in the community‐based doctors and nurses in patient‐centred asthma care: recognition of need and prerequisities to training [Abstract]. American Thoracic Society 2005 International Conference; 2005 May 20‐25; San Diego. 2005:[D93] [Poster: 603]. [CENTRAL: 524613; CRS: 4900100000018663]
-
- Moffat M, Cleland J, Clark N, Cotton, Bucknall C, Griffiths C, et al. An educational intervention for patient‐centred asthma care (PACE) modified for training practice nurses (PNs) and general practitioners (GPs) in Scotland: first stage evaluation [Abstract]. Primary Care Respiratory Journal 2008;17(2):122. [CENTRAL: 642736; CRS: 4900100000022294]
NCT00170248 {published data only}
-
- NCT00170248. Computer‐based decision support in managing asthma in primary care. clinicaltrials.gov/ct2/show/NCT00170248 (first received 13 September 2005).
NCT00214669 {published data only}
-
- NCT00214669. Can education for South Asians with asthma and their clinicians reduce unscheduled care? A randomised trial (OEDIPUS). clinicaltrials.gov/ct2/show/NCT00214669 (first received 14 September 2005).
NCT01522144 {published data only}
-
- NCT01522144. An electronic decision support tool to improve outpatient asthma care. https://clinicaltrials.gov/ct2/show/NCT01522144 (accessed 9 September 2016).
Smith 2008 {published data only}
-
- Smith S, Mitchell C, Bowler S. Standard versus patient‐centred asthma education in the emergency department: a randomised study [see comment]. European Respiratory Journal 2008;31(5):990‐7. [CENTRAL: 637591; CRS: 4900100000022025; EMBASE: 2009045444; PUBMED: 18216062] - PubMed
-
- Smith S, Mitchell C, Fleming M, Bowler S. A randomised control trial (RCT) of patient centred education in emergency department (EDS) [Abstract]. Respirology 2005;10(Suppl):A37. [CENTRAL: 518753; CRS: 4900100000018405]
Sockrider 2001 {published data only}
-
- Sockrider MM, Czyzewski DI, West BL, Pella JJ, Swank PR. Promoting family decision‐making using a pediatric asthma action plan. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A293. [CENTRAL: 394615; CRS: 4900100000012784]
Tapp 2014 {published data only}
-
- NCT02047929. Comparing types of implementation of a shared decision making intervention (ADAPT‐NC) [Comparing traditional and participatory dissemination of a shared decision making intervention]. clinicaltrials.gov/show/NCT02047929 (first received 27 January 2014). [CRS: 4900132000022864]
-
- Tapp H, McWilliams A, Ludden T, Kuhn L, Taylor Y, Alkhazraji T, et al. Comparing traditional and participatory dissemination of a shared decision making intervention (ADAPT‐NC): a cluster randomized trial. Implementation Science 2014;9(1):158. [CENTRAL: 1015317; CRS: 4900126000020910; PUBMED: 25359128] - PMC - PubMed
Tieffenberg 2000 {published data only}
-
- Tieffenberg JA, Wood EI, Alonso A, Tossutti MS, Vicente MF. A randomized field trial of ACINDES: a child‐centered training model for children with chronic illnesses (asthma and epilepsy). Journal of Urban Health 2000;77(2):280‐97. [CENTRAL: 297472; CRS: 4900100000008730; PUBMED: 10856009] - PMC - PubMed
References to studies awaiting assessment
Gagné 2017 {published data only}
-
- Gagné M, Légaré F, Moisan J, Boulet L. Adding a decision aid to asthma education: impact on decisional conflict and appropriate medication usage. Value in Health. 2016; Vol. 19, issue 7:A557.
References to ongoing studies
Federman 2015 {published data only}
-
- Federman AD, Martynenko M, O'Conor R, Kannry J, Karp A, Lurio J, et al. Rationale and design of a comparative effectiveness trial of home‐ and clinic‐based self‐management support coaching for older adults with asthma. Contemporary Clinical Trials 2015;44:103‐11. - PubMed
Hoskins 2013 {published data only}
-
- Hoskins G, Abhyankar P, Taylor AD, Duncan E, Sheikh A, Pinnock H, et al. Goal‐setting intervention in patients with active asthma: protocol for a pilot cluster‐randomised controlled trial. Trials 2013;14(1):289. [CENTRAL: 870965; CRS: 4900100000088569; EMBASE: 2013573201; PUBMED: 24021033] - PMC - PubMed
NCT02516449 {published data only}
-
- NCT02516449. Assessment of shared decision making aids in asthma. clinicaltrials.gov/ct2/show/NCT02516449 (first received 2 July 2015).
Additional references
Adams 2001
Akinbami 2002
-
- Akinbami LJ, Schoendorf KC. Trends in childhood asthma: prevalence, health care utilization, and mortality. Pediatrics 2002;110(2 pt 1):315‐22. - PubMed
Akinbami 2006
-
- Akinbami L, Centers for Disease Control and Prevention National Center for Health Statistics. The state of childhood asthma, United States, 1980‐2005. Advance Data 2006;12(381):1‐24. - PubMed
Asthma UK 2011
-
- Asthma UK. Asthma UK’s Research Strategy: 2011–2016. asthma.org.uk/globalassets/research/research_strategy_2011‐2016.pdf (accessed 11 May 2016).
Butz 2007
Caress 2005
Charles 1997
-
- Charles C, Gafni A, Whelan T. Shared decision‐making in the medical encounter: what does it mean? (or it takes at least two to tango). Social Science and Medicine 1997;44(5):681‐92. - PubMed
Charles 1999
-
- Charles C, Gafni A, Whelen T. Decision‐making in the physician‐patient encounter: revisiting the shared treatment decision‐making model. Social Science and Medicine 1999;49:651‐61. - PubMed
Coulter 2011
-
- Coulter A, Collins A. Making shared decision‐making a reality: no decision about me, without me. kingsfund.org.uk/sites/files/kf/Making‐shared‐decision‐making‐a‐reality‐... (accessed 16 August 2016).
Coxeter 2015
Daly 2016
de Benedictis 2007
-
- Benedictis D, Bush A. The challenge of asthma in adolescence. Pediatric Pulmonology 2007;42:683‐92. - PubMed
Durand 2014
Dwamena 2012
Gibson 2002
GINA 2016
-
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2017. http://ginasthma.org/2017‐gina‐report‐global‐strategy‐for‐asthma‐managem... (accessed 28 February 2017).
Global Asthma Network 2014
-
- Global Asthma Network. The Global Asthma Report 2014. http://globalasthmareport.org/index.php (accessed 3 May 2016).
GRADEpro GDT [Computer program]
-
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 11 May 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guevara 2003
Higgins 2011
-
- Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. http://handbook.cochrane.org/.
Institute of Medicine 2009
-
- Committee On Comparative Effectiveness Research Prioritization. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: National Academy Press, 2009.
Joosten 2008
-
- Joosten EAG, DeFuentes‐Merillas L, Weert GH, Sensky T, Staak CPF, Jong CAJ. Systematic review of the effects of shared decision‐making on patient satisfaction, treatment adherence and health status. Psychotherapy and Psychosomatics 2008;77:219‐26. - PubMed
Légaré 2013
-
- Légaré F, Witteman HO. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Affairs 2013;32(2):276‐84. - PubMed
Légaré 2014
Moher 2009
NHLBI/NAEPP 2007
-
- National Heart, Lung and Blood Institute and the National Asthma Education and Prevention Program. Guidelines for the diagnosis and management of asthma (EPR‐3). Section 3, Component 2: education for a partnership in asthma care. https://nhlbi.nih.gov/files/docs/guidelines/05_sec3_comp2.pdf (accessed 11 May 2016).
NRAD 2014
-
- National Review of Asthma Deaths. Why asthma still kills: the national review of asthma deaths. https://rcplondon.ac.uk/sites/default/files/why‐asthma‐still‐kills‐full‐... (accessed 3 May 2016).
RevMan 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rivera‐Spoljaric 2014
-
- Rivera‐Spoljaric K, Halley M, Wilson SR. Shared clinician‐patient decision‐making about treatment of pediatric asthma: what do we know and how can we use it?. Current Opinions in Allergy and Clinical Immunology 2014;14(2):161‐7. - PubMed
Shay 2015
Sleath 2011
Snyder 2016
-
- Snyder H, Engström J. The antecedents, forms and consequences of patient involvement: a narrative review of the literature. International Journal of Nursing Studies 2016;53:351‐78. - PubMed
Veroff 2013
-
- Veroff D, Marr A, Wennberg DE. Enhanced support for shared decision making reduced costs of care for patients with preference‐sensitive conditions. Health Affairs 2013;32(2):285‐93. - PubMed
Wyatt 2015
-
- Wyatt KD, List B, Brinkman WB, Prutsky Lopez G, Asi N, et al. Shared decision making in pediatrics: a systematic review and meta‐analysis. Systematic Review 2015;51:573–83. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

