Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial
- PMID: 28973074
- PMCID: PMC5833648
- DOI: 10.1001/jamaoncol.2017.3501
Consolidative Radiotherapy for Limited Metastatic Non-Small-Cell Lung Cancer: A Phase 2 Randomized Clinical Trial
Abstract
Importance: Patterns-of-failure studies suggest that in metastatic non-small-cell lung cancer (NSCLC) sites of gross disease at presentation are the first to progress when treated with chemotherapy. This knowledge has led to increased adoption of local ablative radiation therapy in patients with stage IV NSCLC, though prospective randomized evidence is limited.
Objective: To determine if intervening with noninvasive stereotactic ablative radiotherapy (SAbR) prior to maintenance chemotherapy in patients with non-progressive limited metastatic NSCLC after induction therapy led to significant improvements in progression-free survival (PFS).
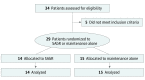
Design, setting, and participants: This is a single-institution randomized phase 2 study of maintenance chemotherapy alone vs SAbR followed by maintenance chemotherapy for patients with limited metastatic NSCLC (primary plus up to 5 metastatic sites) whose tumors did not possess EGFR-targetable or ALK-targetable mutations but did achieve a partial response or stable disease after induction chemotherapy.
Interventions: Maintenance chemotherapy or SAbR to all sites of gross disease (including SAbR or hypofractionated radiation to the primary) followed by maintenance chemotherapy.
Main outcomes and measures: The primary end point was PFS; secondary end points included toxic effects, local and distant tumor control, patterns of failure, and overall survival.
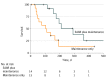
Results: A total of 29 patients (9 women and 20 men) were enrolled; 14 patients (median [range] age, 63.5 [51.0-78.0] years) were allocated to the SAbR-plus-maintenance chemotherapy arm, and 15 patients (median [range] age, 70.0 [51.0-79.0] years) were allocated to the maintenance chemotherapy-alone arm. The trial was stopped to accrual early after an interim analysis found a significant improvement in PFS in the SAbR-plus-maintenance chemotherapy arm of 9.7 months vs 3.5 months in the maintenance chemotherapy-alone arm (P = .01). Toxic effects were similar in both arms. There were no in-field failures with fewer overall recurrences in the SAbR arm while those patients receiving maintenance therapy alone had progression at existing sites of disease and distantly.
Conclusions and relevance: Consolidative SAbR prior to maintenance chemotherapy appeared beneficial, nearly tripling PFS in patients with limited metastatic NSCLC compared with maintenance chemotherapy alone, with no difference in toxic effects. The irradiation prevented local failures in original disease, the most likely sites of first recurrence. Furthermore, PFS for patients with limited metastatic disease appeared similar to those patients with a greater metastatic burden, further arguing for the potential benefits of local therapy in limited metastatic settings.
Trial registration: clinicaltrials.gov Identifier: NCT02045446.
Conflict of interest statement
Figures
Comment in
-
Stereotactic ablative radiotherapy for oligometastatic non-small cell lung cancer.J Thorac Dis. 2018 Jan;10(1):21-24. doi: 10.21037/jtd.2017.11.141. J Thorac Dis. 2018. PMID: 29600013 Free PMC article. No abstract available.
-
Perspectives on oligometastasis: challenges and opportunities.J Thorac Dis. 2018 Jan;10(1):113-117. doi: 10.21037/jtd.2017.12.77. J Thorac Dis. 2018. PMID: 29600035 Free PMC article. No abstract available.
-
Ablative therapy in oligometastatic non-small cell lung cancer-an editorial on recent evidence.J Thorac Dis. 2018 Jan;10(1):138-140. doi: 10.21037/jtd.2017.12.119. J Thorac Dis. 2018. PMID: 29600041 Free PMC article. No abstract available.
-
Consolidative ablative radiotherapy improves outcomes in oligometastatic non-small cell lung cancer: a further step toward new evidence.J Thorac Dis. 2018 Feb;10(2):621-624. doi: 10.21037/jtd.2018.01.19. J Thorac Dis. 2018. PMID: 29607124 Free PMC article. No abstract available.
-
The Objective of Local Therapy in Oligometastatic Cancer Is a Moving Target.JAMA Oncol. 2018 Sep 1;4(9):1296. doi: 10.1001/jamaoncol.2018.1225. JAMA Oncol. 2018. PMID: 29955778 No abstract available.
-
The Objective of Local Therapy in Oligometastatic Cancer Is a Moving Target-Reply.JAMA Oncol. 2018 Sep 1;4(9):1296-1297. doi: 10.1001/jamaoncol.2018.1228. JAMA Oncol. 2018. PMID: 29955791 No abstract available.
-
Oligoreview of Non-Small Cell Lung Cancer Oligometastases.Int J Radiat Oncol Biol Phys. 2020 Mar 1;106(3):455-459. doi: 10.1016/j.ijrobp.2019.11.009. Int J Radiat Oncol Biol Phys. 2020. PMID: 32014142 No abstract available.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. - PubMed
-
- Brodowicz T, Ciuleanu T, Crawford J, et al. ; Central European Cooperative Oncology Group (CECOG) . Third CECOG consensus on the systemic treatment of non-small-cell lung cancer. Ann Oncol. 2012;23(5):1223-1229. - PubMed
-
- Brodowicz T, Krzakowski M, Zwitter M, et al. ; Central European Cooperative Oncology Group CECOG . Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer. 2006;52(2):155-163. - PubMed
-
- Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31(23):2895-2902. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

