Association of Prior Authorization and Out-of-pocket Costs With Patient Access to PCSK9 Inhibitor Therapy
- PMID: 28973087
- PMCID: PMC5963012
- DOI: 10.1001/jamacardio.2017.3451
Association of Prior Authorization and Out-of-pocket Costs With Patient Access to PCSK9 Inhibitor Therapy
Abstract
Importance: Although PCSK9 inhibitors (PCSK9i) were approved in 2015, their high cost has led to strict prior authorization practices and high copays, and use of PSCK9i in clinical practice has been low.
Objective: To evaluate patient access to PCSK9i among those prescribed therapy.
Design, setting, and participants: Using pharmacy transaction data, we evaluated 45 029 patients who were newly prescribed PCSK9i in the United States between August 1, 2015, and July 31, 2016.
Main outcomes and measures: The proportion of PCSK9i prescriptions approved and abandoned (approved but unfilled); multivariable analyses examined factors associated with approval/abandonment including payor, prescriber specialty, pharmacy benefit manager, out-of-pocket cost (copay), clinical diagnoses, lipid-lowering medication use, and low-density lipoprotein cholesterol levels.
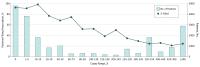
Results: Of patients given an incident PCSK9i prescription, 51.2% were women, 56.6% were 65 years or older, and 52.5% had governmental insurance. Of the patients given a prescription, 20.8% received approval on the first day, and 47.2% ever received approval. Of those approved, 65.3% filled the prescription, resulting in 30.9% of those prescribed PCSK9i ever receiving therapy. After adjustment, patients who were older, male, and had atherosclerotic cardiovascular disease were more likely to be approved, but approval rates did not vary by patient low-density lipoprotein cholesterol level nor statin use. Other factors associated with drug approval included having government vs commercial insurance (odds ratio [OR], 3.3; 95% CI, 2.8-3.8), and those filled at a specialty vs retail pharmacy (OR, 1.96; 95% CI, 1.66-2.33). Approval rates varied nearly 3-fold among the top 10 largest pharmacy benefit managers. Prescription abandonment by patients was most associated with copay costs (C statistic, 0.86); with abandonment rates ranging from 7.5% for those with $0 copay to more than 75% for copays greater than $350.
Conclusions and relevance: In the first year of availability, only half of patients prescribed a PCSK9i received approval, and one-third of approved prescriptions were never filled owing to copay.
Conflict of interest statement
Figures

Comment in
-
Author Relationships With Industry: Policies and Procedures for Authors and Editors of JAMA Cardiology.JAMA Cardiol. 2017 Nov 1;2(11):1181-1182. doi: 10.1001/jamacardio.2017.3461. JAMA Cardiol. 2017. PMID: 28973086 No abstract available.
References
-
- Food and Drug Administration (FDA) BLA approval, BLA 125559. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/125559Orig1.... Published July 24, 2015. Accessed March 28, 2017.
-
- Food and Drug Administration (FDA) BLA approval, BLA 125522/Original 1. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2015/125522Orig1.... Published August 27, 2015. Accessed March 28, 2017.
-
- Kazi DS, Moran AE, Coxson PG, et al. . Cost-effectiveness of PCSK9 inhibitor therapy in patients with heterozygous familial hypercholesterolemia or atherosclerotic cardiovascular disease. JAMA. 2016;316(7):743-753. - PubMed
-
- Mastey V, Johnstone BM. Cost-effectiveness of PCSK9 Inhibitor Therapy. JAMA. 2016;316(20):2151-2152. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

