Homeostatic plasticity can be induced and expressed to restore synaptic strength at neuromuscular junctions undergoing ALS-related degeneration
- PMID: 28973139
- PMCID: PMC5886083
- DOI: 10.1093/hmg/ddx304
Homeostatic plasticity can be induced and expressed to restore synaptic strength at neuromuscular junctions undergoing ALS-related degeneration
Abstract
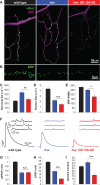
Amyotrophic lateral sclerosis (ALS) is debilitating neurodegenerative disease characterized by motor neuron dysfunction and progressive weakening of the neuromuscular junction (NMJ). Hereditary ALS is strongly associated with variants in the human C9orf72 gene. We have characterized C9orf72 pathology at the Drosophila NMJ and utilized several approaches to restore synaptic strength in this model. First, we demonstrate a dramatic reduction in synaptic arborization and active zone number at NMJs following C9orf72 transgenic expression in motor neurons. Further, neurotransmission is similarly reduced at these synapses, consistent with severe degradation. However, despite these defects, C9orf72 synapses still retain the ability to express presynaptic homeostatic plasticity, a fundamental and adaptive form of NMJ plasticity in which perturbation to postsynaptic neurotransmitter receptors leads to a retrograde enhancement in presynaptic release. Next, we show that these endogenous but dormant homeostatic mechanisms can be harnessed to restore synaptic strength despite C9orf72 pathogenesis. Finally, activation of regenerative signaling is not neuroprotective in motor neurons undergoing C9orf72 toxicity. Together, these experiments define synaptic dysfunction at NMJs experiencing ALS-related degradation and demonstrate the potential to activate latent plasticity as a novel therapeutic strategy to restore synaptic strength.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



Similar articles
-
Post-transcriptional Inhibition of Hsc70-4/HSPA8 Expression Leads to Synaptic Vesicle Cycling Defects in Multiple Models of ALS.Cell Rep. 2017 Oct 3;21(1):110-125. doi: 10.1016/j.celrep.2017.09.028. Cell Rep. 2017. PMID: 28978466 Free PMC article.
-
Motor neuron apoptosis and neuromuscular junction perturbation are prominent features in a Drosophila model of Fus-mediated ALS.Mol Neurodegener. 2012 Mar 24;7:10. doi: 10.1186/1750-1326-7-10. Mol Neurodegener. 2012. PMID: 22443542 Free PMC article.
-
Maintenance of homeostatic plasticity at the Drosophila neuromuscular synapse requires continuous IP3-directed signaling.Elife. 2019 Jun 10;8:e39643. doi: 10.7554/eLife.39643. Elife. 2019. PMID: 31180325 Free PMC article.
-
Synaptic dysfunction and altered excitability in C9ORF72 ALS/FTD.Brain Res. 2018 Aug 15;1693(Pt A):98-108. doi: 10.1016/j.brainres.2018.02.011. Epub 2018 Feb 14. Brain Res. 2018. PMID: 29453960 Free PMC article. Review.
-
Synaptic homeostats: latent plasticity revealed at the Drosophila neuromuscular junction.Cell Mol Life Sci. 2021 Apr;78(7):3159-3179. doi: 10.1007/s00018-020-03732-3. Epub 2021 Jan 15. Cell Mol Life Sci. 2021. PMID: 33449150 Free PMC article. Review.
Cited by
-
Cul3 and insomniac are required for rapid ubiquitination of postsynaptic targets and retrograde homeostatic signaling.Nat Commun. 2019 Jul 5;10(1):2998. doi: 10.1038/s41467-019-10992-6. Nat Commun. 2019. PMID: 31278365 Free PMC article.
-
Levels of Par-1 kinase determine the localization of Bruchpilot at the Drosophila neuromuscular junction synapses.Sci Rep. 2018 Oct 31;8(1):16099. doi: 10.1038/s41598-018-34250-9. Sci Rep. 2018. PMID: 30382129 Free PMC article.
-
The E3 ligase Thin controls homeostatic plasticity through neurotransmitter release repression.Elife. 2022 Jul 7;11:e71437. doi: 10.7554/eLife.71437. Elife. 2022. PMID: 35796533 Free PMC article.
-
Botulinum neurotoxin accurately separates tonic vs. phasic transmission and reveals heterosynaptic plasticity rules in Drosophila.Elife. 2022 Aug 22;11:e77924. doi: 10.7554/eLife.77924. Elife. 2022. PMID: 35993544 Free PMC article.
-
Physiologic and Nanoscale Distinctions Define Glutamatergic Synapses in Tonic vs Phasic Neurons.J Neurosci. 2023 Jun 21;43(25):4598-4611. doi: 10.1523/JNEUROSCI.0046-23.2023. Epub 2023 May 23. J Neurosci. 2023. PMID: 37221096 Free PMC article.
References
-
- Rosen D.R., Siddique T., Patterson D., Figlewicz D.A., Sapp P., Hentati A., Donaldson D., Goto J., O'Regan J.P., Deng H.-X.. et al. (1993) Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature, 362, 59–62. - PubMed
-
- Chew J., Gendron T.F., Prudencio M., Sasaguri H., Zhang Y.-J., Castanedes-Casey M., Lee C.W., Jansen-West K., Kurti A., Murray M.E.. et al. (2015) Neurodegeneration. C9ORF72 repeat expansions in mice cause TDP-43 pathology, neuronal loss, and behavioral deficits. Science, 348, 1151–1154. - PMC - PubMed
-
- Xu Z., Poidevin M., Li X., Li Y., Shu L., Nelson D.L., Li H., Hales C.M., Gearing M., Wingo T.S.. et al. (2013) Expanded GGGGCC repeat RNA associated with amyotrophic lateral sclerosis and frontotemporal dementia causes neurodegeneration. Proc. Natl. Acad. Sci. U. S. A., 110, 7778–7783. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

