Low-Dose vs Standard-Dose Alteplase for Patients With Acute Ischemic Stroke: Secondary Analysis of the ENCHANTED Randomized Clinical Trial
- PMID: 28973174
- PMCID: PMC5822216
- DOI: 10.1001/jamaneurol.2017.2286
Low-Dose vs Standard-Dose Alteplase for Patients With Acute Ischemic Stroke: Secondary Analysis of the ENCHANTED Randomized Clinical Trial
Erratum in
-
Errors in Abstract and Figures 2 and 3.JAMA Neurol. 2018 Mar 1;75(3):384. doi: 10.1001/jamaneurol.2017.4512. JAMA Neurol. 2018. PMID: 29297037 Free PMC article. No abstract available.
Abstract
Importance: A lower dose of intravenous alteplase appears to be a safer treatment option than the standard dose, reducing the risk of symptomatic intracerebral hemorrhage. There is uncertainty, however, over how this effect translates into an overall clinical benefit for patients with acute ischemic stroke (AIS).
Objective: To assess whether older, Asian, or severely affected patients with AIS who are considered at high risk of thrombolysis may benefit more from low-dose rather than standard-dose alteplase treatment.
Design, setting, and participants: This study is a prespecified secondary analysis of the Enhanced Control of Hypertension and Thrombolysis Stroke Study (ENCHANTED), an international, randomized, open-label, blinded, end-point clinical trial of low-dose vs standard-dose intravenous alteplase for patients with AIS. From March 1, 2012, to August 31, 2015, a total of 3310 patients who had a clinical diagnosis of AIS as confirmed by brain imaging and who fulfilled the local criteria for thrombolysis treatment were included in the alteplase-dose arms. Patients were randomly assigned to receive low-dose (0.6 mg/kg; 15% as bolus and 85% as infusion over 1 hour) or standard-dose (0.9 mg/kg; 10% as bolus and 90% as infusion over 1 hour) alteplase. Of the 3310 randomized patients, 13 patients were excluded for missing consent, mistaken randomization, and duplicate randomization numbers. This secondary analysis was conducted between May 1, 2016, and April 28, 2017.
Main outcomes and measures: The primary end point was a poor outcome defined by the combination of death and any disability as scored by the modified Rankin Scale (scores range from 2 to 6, with the highest score indicating death) at 90 days.
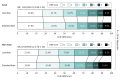
Results: Of the 3297 patients included in the analysis, 1248 (37.9%) were women, and the mean (SD) age was 67 (13) years. No significant differences in the treatment effects were observed between low- and standard-dose alteplase for poor outcomes (death or disability) by age, ethnicity, or severity (all P > .37 for interaction). Similarly, the treatment effects of low- vs standard-dose alteplase on function outcome (ordinal shift of the modified Rankin Scale) in Asians (odds ratio, 1.05; 95% CI, 0.90-1.22) was consistent with non-Asians (odds ratio, 0.93; 95% CI, 0.76-1.14) (P = .32 for interaction). There were generally consistent reductions in rates of symptomatic intracerebral hemorrhage with low-dose alteplase, although this reduction was not statistically significant by age, ethnicity, or severity.
Conclusions and relevance: This analysis found that the effects of low-dose alteplase were not clearly superior to the effects of standard-dose alteplase on death or disability in key demographic subgroups of patients with AIS. Further investigation is required to identify patients with AIS who may benefit from low-dose alteplase.
Trial registration: clinicaltrials.gov Identifier: NCT01422616.
Conflict of interest statement
Figures


References
-
- Yamaguchi T, Mori E, Minematsu K, et al. ; Japan Alteplase Clinical Trial (J-ACT) Group . Alteplase at 0.6 mg/kg for acute ischemic stroke within 3 hours of onset: Japan Alteplase Clinical Trial (J-ACT). Stroke. 2006;37(7):1810-1815. - PubMed
-
- Mori E, Minematsu K, Nakagawara J, Yamaguchi T, Sasaki M, Hirano T; Japan Alteplase Clinical Trial II Group . Effects of 0.6 mg/kg intravenous alteplase on vascular and clinical outcomes in middle cerebral artery occlusion: Japan Alteplase Clinical Trial II (J-ACT II). Stroke. 2010;41(3):461-465. - PubMed
-
- Ueshima S, Matsuo O. The differences in thrombolytic effects of administrated recombinant t-PA between Japanese and Caucasians. Thromb Haemost. 2002;87(3):544-546. - PubMed
-
- Huang Y, Sharma VK, Robinson T, et al. ; ENCHANTED Investigators . Rationale, design, and progress of the ENhanced Control of Hypertension ANd Thrombolysis strokE stuDy (ENCHANTED) trial: an international multicenter 2 × 2 quasi-factorial randomized controlled trial of low- vs. standard-dose rt-PA and early intensive vs. guideline-recommended blood pressure lowering in patients with acute ischaemic stroke eligible for thrombolysis treatment. Int J Stroke. 2015;10(5):778-788. - PubMed
-
- Chao AC, Liu CK, Chen CH, et al. ; Taiwan Thrombolytic Therapy for Acute Ischemic Stroke (TTT-AIS) Study Group . Different doses of recombinant tissue-type plasminogen activator for acute stroke in Chinese patients. Stroke. 2014;45(8):2359-2365. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

