Metabolic, Reproductive, and Neurologic Abnormalities in Agpat1-Null Mice
- PMID: 28973305
- PMCID: PMC5695831
- DOI: 10.1210/en.2017-00511
Metabolic, Reproductive, and Neurologic Abnormalities in Agpat1-Null Mice
Abstract
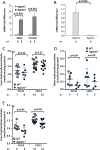
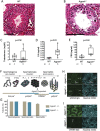
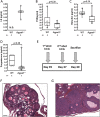
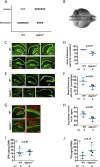
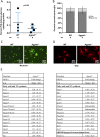
Defects in the biosynthesis of phospholipids and neutral lipids are associated with cell membrane dysfunction, disrupted energy metabolism, and diseases including lipodystrophy. In these pathways, the 1-acylglycerol-3-phosphate O-acyltransferase (AGPAT) enzymes transfer a fatty acid to the sn-2 carbon of sn-1-acylglycerol-3-phosphate (lysophosphatidic acid) to form sn-1, 2-acylglycerol-3-phosphate [phosphatidic acid (PA)]. PA is a precursor for key phospholipids and diacylglycerol. AGPAT1 and AGPAT2 are highly homologous isoenzymes that are both expressed in adipocytes. Genetic defects in AGPAT2 cause congenital generalized lipodystrophy, indicating that AGPAT1 cannot compensate for loss of AGPAT2 in adipocytes. To further explore the physiology of AGPAT1, we characterized a loss-of-function mouse model (Agpat1-/-). The majority of Agpat1-/- mice died before weaning and had low body weight and low plasma glucose levels, independent of plasma insulin and glucagon levels, with reduced percentage of body fat but not generalized lipodystrophy. These mice also had decreased hepatic messenger RNA expression of Igf-1 and Foxo1, suggesting a decrease in gluconeogenesis. In male mice, sperm development was impaired, with a late meiotic arrest near the onset of round spermatid production, and gonadotropins were elevated. Female mice showed oligoanovulation yet retained responsiveness to gonadotropins. Agpat1-/- mice also demonstrated abnormal hippocampal neuron development and developed audiogenic seizures. In summary, Agpat1-/- mice developed widespread disturbances of metabolism, sperm development, and neurologic function resulting from disrupted phospholipid homeostasis. AGPAT1 appears to serve important functions in the physiology of multiple organ systems. The Agpat1-deficient mouse provides an important model in which to study the contribution of phospholipid and triacylglycerol synthesis to physiology and diseases.
Copyright © 2017 Endocrine Society.
Figures




Similar articles
-
Human 1-acylglycerol-3-phosphate O-acyltransferase isoforms 1 and 2: biochemical characterization and inability to rescue hepatic steatosis in Agpat2(-/-) gene lipodystrophic mice.J Biol Chem. 2011 Oct 28;286(43):37676-91. doi: 10.1074/jbc.M111.250449. Epub 2011 Aug 27. J Biol Chem. 2011. PMID: 21873652 Free PMC article.
-
A role for 1-acylglycerol-3-phosphate-O-acyltransferase-1 in myoblast differentiation.Differentiation. 2010 Sep-Oct;80(2-3):140-6. doi: 10.1016/j.diff.2010.05.006. Epub 2010 Jun 19. Differentiation. 2010. PMID: 20561744 Free PMC article.
-
Pathology of congenital generalized lipodystrophy in Agpat2-/- mice.Vet Pathol. 2011 May;48(3):642-54. doi: 10.1177/0300985810383870. Epub 2010 Nov 4. Vet Pathol. 2011. PMID: 21051554
-
Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis.Am J Physiol Endocrinol Metab. 2009 Jun;296(6):E1195-209. doi: 10.1152/ajpendo.90958.2008. Epub 2009 Mar 31. Am J Physiol Endocrinol Metab. 2009. PMID: 19336658 Free PMC article. Review.
-
How lipid droplets "TAG" along: Glycerolipid synthetic enzymes and lipid storage.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 Oct;1862(10 Pt B):1131-1145. doi: 10.1016/j.bbalip.2017.06.010. Epub 2017 Jun 20. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 28642195 Free PMC article. Review.
Cited by
-
AGPAT3 deficiency impairs adipocyte differentiation and leads to a lean phenotype in mice.Am J Physiol Endocrinol Metab. 2024 Jul 1;327(1):E69-E80. doi: 10.1152/ajpendo.00012.2024. Epub 2024 May 8. Am J Physiol Endocrinol Metab. 2024. PMID: 38717361 Free PMC article.
-
BIGKnock: fine-mapping gene-based associations via knockoff analysis of biobank-scale data.Genome Biol. 2023 Feb 13;24(1):24. doi: 10.1186/s13059-023-02864-6. Genome Biol. 2023. PMID: 36782330 Free PMC article.
-
DNA Methylation and Expression Profiles of Whole Blood in Parkinson's Disease.Front Genet. 2021 Apr 26;12:640266. doi: 10.3389/fgene.2021.640266. eCollection 2021. Front Genet. 2021. PMID: 33981329 Free PMC article.
-
Decoding the genetic relationship between Alzheimer's disease and type 2 diabetes: potential risk variants and future direction for North Africa.Front Aging Neurosci. 2023 Jun 5;15:1114810. doi: 10.3389/fnagi.2023.1114810. eCollection 2023. Front Aging Neurosci. 2023. PMID: 37342358 Free PMC article.
-
Aging-Associated Alterations in Mammary Epithelia and Stroma Revealed by Single-Cell RNA Sequencing.Cell Rep. 2020 Dec 29;33(13):108566. doi: 10.1016/j.celrep.2020.108566. Cell Rep. 2020. PMID: 33378681 Free PMC article.
References
-
- Kennedy EP. The synthesis of cytidine diphosphate choline, cytidine diphosphate ethanolamine, and related compounds. J Biol Chem. 1956;222(1):185–191. - PubMed
-
- Donkor J, Sariahmetoglu M, Dewald J, Brindley DN, Reue K. Three mammalian lipins act as phosphatidate phosphatases with distinct tissue expression patterns. J Biol Chem. 2007;282(6):3450–3457. - PubMed
-
- Temprano A, Sembongi H, Han GS, Sebastián D, Capellades J, Moreno C, Guardiola J, Wabitsch M, Richart C, Yanes O, Zorzano A, Carman GM, Siniossoglou S, Miranda M. Redundant roles of the phosphatidate phosphatase family in triacylglycerol synthesis in human adipocytes. Diabetologia. 2016;59(9):1985–1994. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

