Impaired axonal retrograde trafficking of the retromer complex augments lysosomal deficits in Alzheimer's disease neurons
- PMID: 28973312
- PMCID: PMC5886073
- DOI: 10.1093/hmg/ddx321
Impaired axonal retrograde trafficking of the retromer complex augments lysosomal deficits in Alzheimer's disease neurons
Abstract
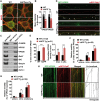
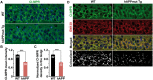
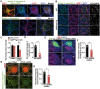
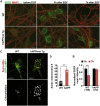
Lysosomal proteolysis is essential for the quality control of intracellular components and the maintenance of cellular homeostasis. Lysosomal alterations have been implicated as one of the main cellular defects contributing to the onset and progression of Alzheimer's disease (AD). However, the mechanism underlying lysosomal deficits in AD remains poorly understood. Here, we reveal that lysosomal deficits are attributed to retromer dysfunction induced by altered retromer trafficking in the axon of AD-related mutant human amyloid precursor protein (hAPP) transgenic (Tg) mouse neurons. We demonstrate that retrograde transport of retromer is impaired, leading to its significant reduction in the soma and abnormal retention within late endosomes in distal axons of mutant hAPP neurons. Therefore, retromer-mediated endosome-to-Golgi retrieval of cation-independent mannose-6-phosphate receptors (CI-MPR) in the soma is disrupted in mutant hAPP neurons, causing defects in lysosome biogenesis. Such defects result in protease deficiency in lysosomes and impaired lysosomal proteolysis, as evidenced by aberrant accumulation of sequestered substrates within lysosomes. Intriguingly, enhancement of retrograde transport in mutant hAPP neurons facilitates the trafficking of axonal retromer toward the soma and thus enhances protease transport to lysosomes, thereby restoring lysosomal proteolytic activity. Taken together, our study provides new insights into the regulation of retromer trafficking through retrograde axonal transport to fulfil its function in promoting lysosome biogenesis in the soma, suggesting a potential approach for rescuing lysosomal proteolysis deficits in AD.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures



Similar articles
-
Regulation of Synaptic Amyloid-β Generation through BACE1 Retrograde Transport in a Mouse Model of Alzheimer's Disease.J Neurosci. 2017 Mar 8;37(10):2639-2655. doi: 10.1523/JNEUROSCI.2851-16.2017. Epub 2017 Feb 3. J Neurosci. 2017. PMID: 28159908 Free PMC article.
-
Autophagy-mediated Regulation of BACE1 Protein Trafficking and Degradation.J Biol Chem. 2017 Feb 3;292(5):1679-1690. doi: 10.1074/jbc.M116.766584. Epub 2016 Dec 27. J Biol Chem. 2017. PMID: 28028177 Free PMC article.
-
The location and trafficking routes of the neuronal retromer and its role in amyloid precursor protein transport.Neurobiol Dis. 2012 Jul;47(1):126-34. doi: 10.1016/j.nbd.2012.03.030. Epub 2012 Apr 6. Neurobiol Dis. 2012. PMID: 22516235 Free PMC article.
-
Sorting receptor SORLA--a trafficking path to avoid Alzheimer disease.J Cell Sci. 2013 Jul 1;126(Pt 13):2751-60. doi: 10.1242/jcs.125393. Epub 2013 Jun 26. J Cell Sci. 2013. PMID: 23813966 Review.
-
Β-site APP-cleaving enzyme 1 trafficking and Alzheimer's disease pathogenesis.J Neurochem. 2012 Mar;120(6):869-80. doi: 10.1111/j.1471-4159.2011.07623.x. Epub 2012 Jan 23. J Neurochem. 2012. PMID: 22171895 Review.
Cited by
-
The emerging role of autophagy and mitophagy in tauopathies: From pathogenesis to translational implications in Alzheimer's disease.Front Aging Neurosci. 2022 Oct 17;14:1022821. doi: 10.3389/fnagi.2022.1022821. eCollection 2022. Front Aging Neurosci. 2022. PMID: 36325189 Free PMC article. Review.
-
Regulation of neuronal autophagy and the implications in neurodegenerative diseases.Neurobiol Dis. 2022 Jan;162:105582. doi: 10.1016/j.nbd.2021.105582. Epub 2021 Dec 7. Neurobiol Dis. 2022. PMID: 34890791 Free PMC article. Review.
-
Mitophagy in Alzheimer's Disease and Other Age-Related Neurodegenerative Diseases.Cells. 2020 Jan 8;9(1):150. doi: 10.3390/cells9010150. Cells. 2020. PMID: 31936292 Free PMC article. Review.
-
De novo macrocyclic peptides for inhibiting, stabilizing, and probing the function of the retromer endosomal trafficking complex.Sci Adv. 2021 Dec 3;7(49):eabg4007. doi: 10.1126/sciadv.abg4007. Epub 2021 Dec 1. Sci Adv. 2021. PMID: 34851660 Free PMC article.
-
Autophagy in alzheimer disease pathogenesis and its therapeutic values.Autophagy Rep. 2025 May 8;4(1):2471677. doi: 10.1080/27694127.2025.2471677. eCollection 2025. Autophagy Rep. 2025. PMID: 40395993 Free PMC article. Review.
References
-
- Luzio J.P., Pryor P.R., Bright N.A. (2007) Lysosomes: fusion and function. Nat. Rev. Mol. Cell. Biol., 8, 622–632. - PubMed
-
- Saftig P., Klumperman J. (2009) Lysosome biogenesis and lysosomal membrane proteins: trafficking meets function. Nat. Rev. Mol. Cell. Biol., 10, 623–635. - PubMed
-
- Saftig P., Haas A. (2016) Turn up the lysosome. Nat. Cell. Biol., 18, 1025–1027. - PubMed
-
- Nixon R.A. (2005) Endosome function and dysfunction in Alzheimer's disease and other neurodegenerative diseases. Neurobiol. Aging, 26, 373–382. - PubMed
-
- Nixon R.A., Cataldo A.M. (2006) Lysosomal system pathways: genes to neurodegeneration in Alzheimer's disease. J. Alzheimers Dis., 9, 277–289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

