Mechanism of opening a sliding clamp
- PMID: 28973453
- PMCID: PMC5737080
- DOI: 10.1093/nar/gkx665
Mechanism of opening a sliding clamp
Abstract
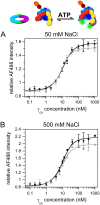
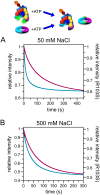
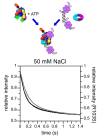
Clamp loaders load ring-shaped sliding clamps onto DNA where the clamps serve as processivity factors for DNA polymerases. In the first stage of clamp loading, clamp loaders bind and stabilize clamps in an open conformation, and in the second stage, clamp loaders place the open clamps around DNA so that the clamps encircle DNA. Here, the mechanism of the initial clamp opening stage is investigated. Mutations were introduced into the Escherichia coli β-sliding clamp that destabilize the dimer interface to determine whether the formation of an open clamp loader-clamp complex is dependent on spontaneous clamp opening events. In other work, we showed that mutation of a positively charged Arg residue at the β-dimer interface and high NaCl concentrations destabilize the clamp, but neither facilitates the formation of an open clamp loader-clamp complex in experiments presented here. Clamp opening reactions could be fit to a minimal three-step 'bind-open-lock' model in which the clamp loader binds a closed clamp, the clamp opens, and subsequent conformational rearrangements 'lock' the clamp loader-clamp complex in a stable open conformation. Our results support a model in which the E. coli clamp loader actively opens the β-sliding clamp.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




Similar articles
-
The Escherichia coli clamp loader can actively pry open the β-sliding clamp.J Biol Chem. 2011 Dec 9;286(49):42704-42714. doi: 10.1074/jbc.M111.268169. Epub 2011 Oct 4. J Biol Chem. 2011. PMID: 21971175 Free PMC article.
-
Solution structure of an "open" E. coli Pol III clamp loader sliding clamp complex.J Struct Biol. 2016 Jun;194(3):272-81. doi: 10.1016/j.jsb.2016.03.003. Epub 2016 Mar 8. J Struct Biol. 2016. PMID: 26968362
-
ATP binding to the Escherichia coli clamp loader powers opening of the ring-shaped clamp of DNA polymerase III holoenzyme.J Biol Chem. 1998 Sep 18;273(38):24550-63. doi: 10.1074/jbc.273.38.24550. J Biol Chem. 1998. PMID: 9733750
-
Dynamics of loading the Escherichia coli DNA polymerase processivity clamp.Crit Rev Biochem Mol Biol. 2006 May-Jun;41(3):179-208. doi: 10.1080/10409230600648751. Crit Rev Biochem Mol Biol. 2006. PMID: 16760017 Review.
-
Replication clamps and clamp loaders.Cold Spring Harb Perspect Biol. 2013 Apr 1;5(4):a010165. doi: 10.1101/cshperspect.a010165. Cold Spring Harb Perspect Biol. 2013. PMID: 23545418 Free PMC article. Review.
Cited by
-
The Macromolecular Machines that Duplicate the Escherichia coli Chromosome as Targets for Drug Discovery.Antibiotics (Basel). 2018 Mar 14;7(1):23. doi: 10.3390/antibiotics7010023. Antibiotics (Basel). 2018. PMID: 29538288 Free PMC article. Review.
-
The bacterial DNA sliding clamp, β-clamp: structure, interactions, dynamics and drug discovery.Cell Mol Life Sci. 2024 May 30;81(1):245. doi: 10.1007/s00018-024-05252-w. Cell Mol Life Sci. 2024. PMID: 38814467 Free PMC article. Review.
-
Allosteric communication in DNA polymerase clamp loaders relies on a critical hydrogen-bonded junction.Elife. 2021 Apr 13;10:e66181. doi: 10.7554/eLife.66181. Elife. 2021. PMID: 33847559 Free PMC article.
-
The SOS Error-Prone DNA Polymerase V Mutasome and β-Sliding Clamp Acting in Concert on Undamaged DNA and during Translesion Synthesis.Cells. 2021 May 1;10(5):1083. doi: 10.3390/cells10051083. Cells. 2021. PMID: 34062858 Free PMC article.
-
Potassium Glutamate and Glycine Betaine Induce Self-Assembly of the PCNA and β-Sliding Clamps.Biophys J. 2021 Jan 5;120(1):73-85. doi: 10.1016/j.bpj.2020.11.013. Epub 2020 Nov 19. Biophys J. 2021. PMID: 33221249 Free PMC article.
References
-
- Piperno J.R., Alberts B.M.. An ATP stimulation of T4 DNA polymerase mediated via T4 gene 44/62 and 45 proteins. The requirement for ATP hydrolysis. J. Biol. Chem. 1978; 253:5174–5179. - PubMed
-
- Fay P.J., Johanson K.O., McHenry C.S., Bambara R.A.. Size classes of products synthesized processively by two subassemblies of Escherichia coli DNA polymerase III holoenzyme. J. Biol. Chem. 1982; 257:5692–5699. - PubMed
-
- Vivona J.B., Kelman Z.. The diverse spectrum of sliding clamp interacting proteins. FEBS Lett. 2003; 546:167–172. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

