An RNA-targeted therapy for dystrophic epidermolysis bullosa
- PMID: 28973459
- PMCID: PMC5737646
- DOI: 10.1093/nar/gkx669
An RNA-targeted therapy for dystrophic epidermolysis bullosa
Abstract
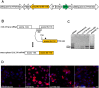
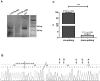
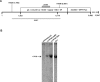
Functional impairment or complete loss of type VII collagen, caused by mutations within COL7A1, lead to the severe recessive form of the skin blistering disease dystrophic epidermolysis bullosa (RDEB). Here, we successfully demonstrate RNA trans-splicing as an auspicious repair option for mutations located in a wide range of exons by fully converting an RDEB phenotype in an ex vivo pre-clinical mouse model based on xenotransplantation. Via a self-inactivating (SIN) lentiviral vector a 3' RNA trans-splicing molecule, capable of replacing COL7A1 exons 65-118, was delivered into type VII collagen deficient patient keratinocytes, carrying a homozygous mutation in exon 80 (c.6527insC). Following vector integration, protein analysis of an isolated corrected single cell clone showed secretion of the corrected type VII collagen at similar levels compared to normal keratinocytes. To confirm full phenotypic and long-term correction in vivo, patches of skin equivalents expanded from the corrected cell clone were grafted onto immunodeficient mice. Immunolabelling of 12 weeks old skin specimens showed strong expression of human type VII collagen restricted to the basement membrane zone. We demonstrate that the RNA trans-splicing technology combined with a SIN lentiviral vector is suitable for an ex vivo molecular therapy approach and thus adaptable for clinical application.
© The Author(s) 2017. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



Similar articles
-
Functional correction of type VII collagen expression in dystrophic epidermolysis bullosa.J Invest Dermatol. 2011 Jan;131(1):74-83. doi: 10.1038/jid.2010.249. Epub 2010 Aug 19. J Invest Dermatol. 2011. PMID: 20720561
-
COL7A1 Editing via CRISPR/Cas9 in Recessive Dystrophic Epidermolysis Bullosa.Mol Ther. 2017 Nov 1;25(11):2573-2584. doi: 10.1016/j.ymthe.2017.07.005. Epub 2017 Jul 13. Mol Ther. 2017. PMID: 28800953 Free PMC article.
-
Intradermal injection of lentiviral vectors corrects regenerated human dystrophic epidermolysis bullosa skin tissue in vivo.Mol Ther. 2004 Aug;10(2):318-26. doi: 10.1016/j.ymthe.2004.05.016. Mol Ther. 2004. PMID: 15294178
-
Gene therapy: pursuing restoration of dermal adhesion in recessive dystrophic epidermolysis bullosa.Exp Dermatol. 2014 Jan;23(1):1-6. doi: 10.1111/exd.12246. Exp Dermatol. 2014. PMID: 24107073 Review.
-
[Recessive dystrophic bullous epidermolysis: Is the etiological treatment at the streetcorner?].Ann Dermatol Venereol. 2011 Oct;138(10):710-1. doi: 10.1016/j.annder.2011.05.002. Epub 2011 Jun 8. Ann Dermatol Venereol. 2011. PMID: 21978513 Review. French. No abstract available.
Cited by
-
5'RNA Trans-Splicing Repair of COL7A1 Mutant Transcripts in Epidermolysis Bullosa.Int J Mol Sci. 2022 Feb 2;23(3):1732. doi: 10.3390/ijms23031732. Int J Mol Sci. 2022. PMID: 35163654 Free PMC article.
-
Improved Double-Nicking Strategies for COL7A1-Editing by Homologous Recombination.Mol Ther Nucleic Acids. 2019 Dec 6;18:496-507. doi: 10.1016/j.omtn.2019.09.011. Epub 2019 Sep 20. Mol Ther Nucleic Acids. 2019. PMID: 31670199 Free PMC article.
-
Molecular Therapeutics in Development for Epidermolysis Bullosa: Update 2020.Mol Diagn Ther. 2020 Jun;24(3):299-309. doi: 10.1007/s40291-020-00466-7. Mol Diagn Ther. 2020. PMID: 32328988 Free PMC article. Review.
-
Gene-edited cells: novel allogeneic gene/cell therapy for epidermolysis bullosa.J Appl Genet. 2024 Dec;65(4):705-726. doi: 10.1007/s13353-024-00839-2. Epub 2024 Mar 9. J Appl Genet. 2024. PMID: 38459407 Review.
-
Integrated Management Strategies for Epidermolysis Bullosa: Current Insights.Int J Gen Med. 2022 May 24;15:5133-5144. doi: 10.2147/IJGM.S342740. eCollection 2022. Int J Gen Med. 2022. PMID: 35637703 Free PMC article. Review.
References
-
- Fine J.D., Hintner H.. Life with Epidermolysis Bullosa (EB): Etiology, Diagnosis, Multicisciplinary Care and Therapy. 2009.
-
- Weber F., Bauer J.W., Sepp N., Hogler W., Salmhofer W., Hintner H., Fritsch P.. Squamous cell carcinoma in junctional and dystrophic epidermolysis bullosa. Acta Derm. Venereol. 2001; 81:189–192. - PubMed
-
- Siprashvili Z., Nguyen N.T., Gorell E.S., Loutit K., Khuu P., Furukawa L.K., Lorenz H.P., Leung T.H., Keene D.R., Rieger K.E. et al. . Safety and wound outcomes following genetically corrected autologous epidermal grafts in patients with recessive dystrophic Epidermolysis bullosa. JAMA. 2016; 316:1808–1817. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

