Longitudinal Hemodynamics of Transcatheter and Surgical Aortic Valves in the PARTNER Trial
- PMID: 28973520
- PMCID: PMC5710363
- DOI: 10.1001/jamacardio.2017.3306
Longitudinal Hemodynamics of Transcatheter and Surgical Aortic Valves in the PARTNER Trial
Abstract
Importance: Use of transcatheter aortic valve replacement (TAVR) for severe aortic stenosis is growing rapidly. However, to our knowledge, the durability of these prostheses is incompletely defined.
Objective: To determine the midterm hemodynamic performance of balloon-expandable transcatheter heart valves.
Design, setting, and participants: In this study, we analyzed core laboratory-generated data from echocardiograms of all patients enrolled in the Placement of Aortic Transcatheter Valves (PARTNER) 1 Trial with successful TAVR or surgical AVR (SAVR) obtained preimplantation and at 7 days, 1 and 6 months, and 1, 2, 3, 4, and 5 years postimplantation. Patients from continued access observational studies were included for comparison.
Interventions: Successful implantation after randomization to TAVR vs SAVR (PARTNER 1A; TAVR, n = 321; SAVR, n = 313), TAVR vs medical treatment (PARTNER 1B; TAVR, n = 165), and continued access (TAVR, n = 1996). Five-year echocardiogram data were available for 424 patients after TAVR and 49 after SAVR.
Main outcomes and measures: Death or reintervention for aortic valve structural indications, measured using aortic valve mean gradient, effective orifice area, Doppler velocity index, and evidence of hemodynamic deterioration by reintervention, adverse hemodynamics, or transvalvular regurgitation.
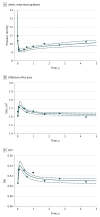
Results: Of 2795 included patients, the mean (SD) age was 84.5 (7.1) years, and 1313 (47.0%) were female. Population hemodynamic trends derived from nonlinear mixed-effects models showed small early favorable changes in the first few months post-TAVR, with a decrease of -2.9 mm Hg in aortic valve mean gradient, an increase of 0.028 in Doppler velocity index, and an increase of 0.09 cm2 in effective orifice area. There was relative stability at a median follow-up of 3.1 (maximum, 5) years. Moderate/severe transvalvular regurgitation was noted in 89 patients (3.7%) after TAVR and increased over time. Patients with SAVR showed no significant changes. In TAVR, death/reintervention was associated with lower ejection fraction, stroke volume index, and aortic valve mean gradient up to 3 years, with no association with Doppler velocity index or valve area. Reintervention occurred in 20 patients (0.8%) after TAVR and in 1 (0.3%) after SAVR and became less frequent over time. Reintervention was caused by structural deterioration of transcatheter heart valves in only 5 patients. Severely abnormal hemodynamics on echocardiograms were also infrequent and not associated with excess death or reintervention for either TAVR or SAVR.
Conclusions and relevance: This large, core laboratory-based study of transcatheter heart valves revealed excellent durability of the transcatheter heart valves and SAVR. Abnormal findings in individual patients, suggestive of valve thrombosis or structural deterioration, were rare in this protocol-driven database and require further investigation.
Trial registration: clinicaltrials.gov Identifier: NCT00530894.
Conflict of interest statement
Figures

Comment in
-
Will Transcatheter Aortic Valve Replacement Echo Surgical Aortic Valve Replacement Durability?JAMA Cardiol. 2017 Nov 1;2(11):1206-1207. doi: 10.1001/jamacardio.2017.3307. JAMA Cardiol. 2017. PMID: 28973160 No abstract available.
References
-
- Mack MJ, Leon MB, Smith CR, et al. ; PARTNER 1 Trial Investigators . 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2477-2484. - PubMed
-
- Kapadia SR, Leon MB, Makkar RR, et al. ; PARTNER Trial Investigators . 5-year outcomes of transcatheter aortic valve replacement compared with standard treatment for patients with inoperable aortic stenosis (PARTNER 1): a randomised controlled trial. Lancet. 2015;385(9986):2485-2491. - PubMed
-
- Deeb GM, Reardon MJ, Chetcuti S, et al. ; CoreValve US Clinical Investigators . 3-Year outcomes in high-risk patients who underwent surgical or transcatheter aortic valve replacement. J Am Coll Cardiol. 2016;67(22):2565-2574. - PubMed
-
- Brennan JM, Edwards FH, Zhao Y, et al. ; DEcIDE AVR (Developing Evidence to Inform Decisions about Effectiveness–Aortic Valve Replacement) Research Team . Long-term safety and effectiveness of mechanical versus biologic aortic valve prostheses in older patients: results from the Society of Thoracic Surgeons Adult Cardiac Surgery National Database. Circulation. 2013;127(16):1647-1655. - PubMed
-
- Zhao DF, Seco M, Wu JJ, et al. Mechanical versus bioprosthetic aortic valve replacement in middle-aged adults: a systematic review and meta-analysis. Ann Thorac Surg. 2016;102(1):315-327. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

