Loss of the imprinted, non-coding Snord116 gene cluster in the interval deleted in the Prader Willi syndrome results in murine neuronal and endocrine pancreatic developmental phenotypes
- PMID: 28973544
- PMCID: PMC5815655
- DOI: 10.1093/hmg/ddx342
Loss of the imprinted, non-coding Snord116 gene cluster in the interval deleted in the Prader Willi syndrome results in murine neuronal and endocrine pancreatic developmental phenotypes
Abstract
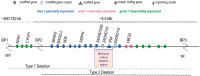
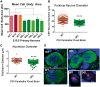
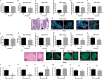
Global neurodevelopmental delay is a prominent characteristic of individuals with Prader-Willi syndrome (PWS). The neuromolecular bases for these delays are unknown. We identified neuroanatomical changes in the brains of mice deficient for a gene in the minimal critical deletion region for PWS (Snord116p-/m+). In Snord116p-/m+ mice, reduced primary forebrain neuron cell body size is apparent in embryonic day 15.5 fetuses, and persists until postnatal day 30 in cerebellar Purkinje neurons. Snord116 is a snoRNA gene cluster of unknown function that can localize to the nucleolus. In cerebellar Purkinje neurons from postnatal day 30 Snord116p-/m+ mice the reduction in neuronal cell body size was associated with decreased neuronal nucleolar size. We also identified developmental changes in the endocrine pancreas of Snord116p-/m+ animals that persist into adulthood. Mice lacking Snord116 have smaller pancreatic islets; within the islet the percentage of δ-cells is increased, while the percentage of α-cells is reduced. The α-cell markers, Sst and Hhex, are upregulated in Snord116p-/m+ isolated islets while Ins1, Ins2, Pdx1, Nkx6-1, and Pax6 are downregulated. There is a 3-fold increase in the percentage of polyhormonal cells in the neonatal pancreata of Snord116p-/m+ mice, due primarily to an increase in cells co-positive with somatostatin. Snord116 may play a role in islet cell lineage specification. The Snord116 gene cluster is important for developmental processes in the brain as well as the endocrine pancreas.
© The Author 2017. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Butler M.G., Lee P.D.K., Whitman B.Y. (2006) Management of Prader-Willi Syndrome. Springer Science Business Media, Inc, New York.
-
- Miller J.L., Couch J.A., Schmalfuss I., He G., Liu Y., Driscoll D.J. (2007) Intracranial abnormalities detected by three-dimensional magnetic resonance imaging in Prader-Willi syndrome. Am. J. Med. Genet. A, 143A, 476–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

