Effect of Routine Low-Dose Oxygen Supplementation on Death and Disability in Adults With Acute Stroke: The Stroke Oxygen Study Randomized Clinical Trial
- PMID: 28973619
- PMCID: PMC5818819
- DOI: 10.1001/jama.2017.11463
Effect of Routine Low-Dose Oxygen Supplementation on Death and Disability in Adults With Acute Stroke: The Stroke Oxygen Study Randomized Clinical Trial
Erratum in
-
Incorrect Statistical Measures and Typographical Errors.JAMA. 2017 Nov 14;318(18):1831-1832. doi: 10.1001/jama.2017.15984. JAMA. 2017. PMID: 29136427 Free PMC article. No abstract available.
Abstract
Importance: Hypoxia is common in the first few days after acute stroke, is frequently intermittent, and is often undetected. Oxygen supplementation could prevent hypoxia and secondary neurological deterioration and thus has the potential to improve recovery.
Objective: To assess whether routine prophylactic low-dose oxygen therapy was more effective than control oxygen administration in reducing death and disability at 90 days, and if so, whether oxygen given at night only, when hypoxia is most frequent, and oxygen administration is least likely to interfere with rehabilitation, was more effective than continuous supplementation.
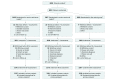
Design, setting, and participants: In this single-blind randomized clinical trial, 8003 adults with acute stroke were enrolled from 136 participating centers in the United Kingdom within 24 hours of hospital admission if they had no clear indications for or contraindications to oxygen treatment (first patient enrolled April 24, 2008; last follow-up January 27, 2015).
Interventions: Participants were randomized 1:1:1 to continuous oxygen for 72 hours (n = 2668), nocturnal oxygen (21:00 to 07:00 hours) for 3 nights (n = 2667), or control (oxygen only if clinically indicated; n = 2668). Oxygen was given via nasal tubes at 3 L/min if baseline oxygen saturation was 93% or less and at 2 L/min if oxygen saturation was greater than 93%.
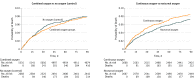
Main outcomes and measures: The primary outcome was reported using the modified Rankin Scale score (disability range, 0 [no symptoms] to 6 [death]; minimum clinically important difference, 1 point), assessed at 90 days by postal questionnaire (participant aware, assessor blinded). The modified Rankin Scale score was analyzed by ordinal logistic regression, which yields a common odds ratio (OR) for a change from one disability level to the next better (lower) level; OR greater than 1.00 indicates improvement.
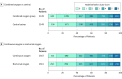
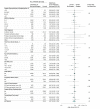
Results: A total of 8003 patients (4398 (55%) men; mean [SD] age, 72 [13] years; median National Institutes of Health Stroke Scale score, 5; mean baseline oxygen saturation, 96.6%) were enrolled. The primary outcome was available for 7677 (96%) participants. The unadjusted OR for a better outcome (calculated via ordinal logistic regression) was 0.97 (95% CI, 0.89 to 1.05; P = .47) for oxygen vs control, and the OR was 1.03 (95% CI, 0.93 to 1.13; P = .61) for continuous vs nocturnal oxygen. No subgroup could be identified that benefited from oxygen. At least 1 serious adverse event occurred in 348 (13.0%) participants in the continuous oxygen group, 294 (11.0%) in the nocturnal group, and 322 (12.1%) in the control group. No significant harms were identified.
Conclusions and relevance: Among nonhypoxic patients with acute stroke, the prophylactic use of low-dose oxygen supplementation did not reduce death or disability at 3 months. These findings do not support low-dose oxygen in this setting.
Trial registration: ISRCTN Identifier: ISRCTN52416964.
Conflict of interest statement
Figures
Comment in
-
Prophylactic Low-Dose Oxygen for Patients With Acute Stroke.JAMA. 2018 Feb 6;319(5):501-502. doi: 10.1001/jama.2017.20342. JAMA. 2018. PMID: 29411026 No abstract available.
-
Another Look into Oxygen Supplementation in the Acute Care Setting.Am J Respir Crit Care Med. 2020 Feb 15;201(4):478-480. doi: 10.1164/rccm.201905-1029RR. Am J Respir Crit Care Med. 2020. PMID: 31816250 No abstract available.
References
-
- Roffe C, Sills S, Halim M, et al. Unexpected nocturnal hypoxia in patients with acute stroke. Stroke. 2003;34(11):2641-2645. - PubMed
-
- Rocco A, Pasquini M, Cecconi E, et al. Monitoring after the acute stage of stroke. Stroke. 2007;38(4):1225-1228. - PubMed
-
- Bravata DM, Wells CK, Lo AC, et al. Processes of care associated with acute stroke outcomes. Arch Intern Med. 2010;170(9):804-810. - PubMed
-
- Rowat AM, Dennis MS, Wardlaw JM. Hypoxaemia in acute stroke is frequent and worsens outcome. Cerebrovasc Dis. 2006;21(3):166-172. - PubMed
-
- Heiss WD. The ischemic penumbra: how does tissue injury evolve? Ann N Y Acad Sci. 2012;1268:26-34. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

