Catheterization alters bladder ecology to potentiate Staphylococcus aureus infection of the urinary tract
- PMID: 28973850
- PMCID: PMC5642702
- DOI: 10.1073/pnas.1707572114
Catheterization alters bladder ecology to potentiate Staphylococcus aureus infection of the urinary tract
Abstract
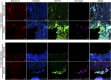
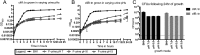
Methicillin-resistant Staphylococcus aureus (MRSA) is an emerging cause of catheter-associated urinary tract infection (CAUTI), which frequently progresses to more serious invasive infections. We adapted a mouse model of CAUTI to investigate how catheterization increases an individual's susceptibility to MRSA UTI. This analysis revealed that catheterization was required for MRSA to achieve high-level, persistent infection in the bladder. As shown previously, catheter placement induced an inflammatory response resulting in the release of the host protein fibrinogen (Fg), which coated the bladder and implant. Following infection, we showed that MRSA attached to the urothelium and implant in patterns that colocalized with deposited Fg. Furthermore, MRSA exacerbated the host inflammatory response to stimulate the additional release and accumulation of Fg in the urinary tract, which facilitated MRSA colonization. Consistent with this model, analysis of catheters from patients with S. aureus-positive cultures revealed colocalization of Fg, which was deposited on the catheter, with S. aureus Clumping Factors A and B (ClfA and ClfB) have been shown to contribute to MRSA-Fg interactions in other models of disease. We found that mutants in clfA had significantly greater Fg-binding defects than mutants in clfB in several in vitro assays. Paradoxically, only the ClfB- strain was significantly attenuated in the CAUTI model. Together, these data suggest that catheterization alters the urinary tract environment to promote MRSA CAUTI pathogenesis by inducing the release of Fg, which the pathogen enhances to persist in the urinary tract despite the host's robust immune response.
Keywords: ClfB–fibrinogen interactions; MRSA CAUTI; host–pathogen interactions.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Barrett SP, et al. Antibiotic sensitivity of bacteria associated with community-acquired urinary tract infection in Britain. J Antimicrob Chemother. 1999;44:359–365. - PubMed
-
- Zhanel GG, et al. NAUTICA Group Antibiotic resistance in outpatient urinary isolates: Final results from the North American Urinary Tract Infection Collaborative Alliance (NAUTICA) Int J Antimicrob Agents. 2005;26:380–388. - PubMed
-
- Al Mohajer M, Musher DM, Minard CG, Darouiche RO. Clinical significance of Staphylococcus aureus bacteriuria at a tertiary care hospital. Scand J Infect Dis. 2013;45:688–695. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

