Speed regulation of genetic cascades allows for evolvability in the body plan specification of insects
- PMID: 28973882
- PMCID: PMC5642680
- DOI: 10.1073/pnas.1702478114
Speed regulation of genetic cascades allows for evolvability in the body plan specification of insects
Abstract
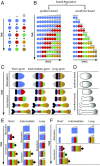
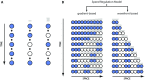
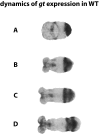
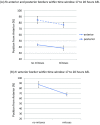
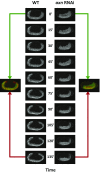
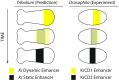
During the anterior-posterior fate specification of insects, anterior fates arise in a nonelongating tissue (called the "blastoderm"), and posterior fates arise in an elongating tissue (called the "germband"). However, insects differ widely in the extent to which anterior-posterior fates are specified in the blastoderm versus the germband. Here we present a model in which patterning in both the blastoderm and germband of the beetle Tribolium castaneum is based on the same flexible mechanism: a gradient that modulates the speed of a genetic cascade of gap genes, resulting in the induction of sequential kinematic waves of gap gene expression. The mechanism is flexible and capable of patterning both elongating and nonelongating tissues, and hence converting blastodermal to germband fates and vice versa. Using RNAi perturbations, we found that blastodermal fates could be shifted to the germband, and germband fates could be generated in a blastoderm-like morphology. We also suggest a molecular mechanism underlying our model, in which gradient levels regulate the switch between two enhancers: One enhancer is responsible for sequential gene activation, and the other is responsible for freezing temporal rhythms into spatial patterns. This model is consistent with findings in Drosophila melanogaster, where gap genes were found to be regulated by two nonredundant "shadow" enhancers.
Keywords: cascade; clock-and-wavefront; enhancer switching; evolution; kinematic waves.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











Similar articles
-
A segmentation clock operating in blastoderm and germband stages of Tribolium development.Development. 2012 Dec 1;139(23):4341-6. doi: 10.1242/dev.085126. Epub 2012 Oct 24. Development. 2012. PMID: 23095886 Free PMC article.
-
A re-inducible gap gene cascade patterns the anterior-posterior axis of insects in a threshold-free fashion.Elife. 2018 Dec 20;7:e41208. doi: 10.7554/eLife.41208. Elife. 2018. PMID: 30570485 Free PMC article.
-
Speeding up anterior-posterior patterning of insects by differential initialization of the gap gene cascade.Dev Biol. 2020 Apr 1;460(1):20-31. doi: 10.1016/j.ydbio.2019.04.015. Epub 2019 May 7. Dev Biol. 2020. PMID: 31075221
-
From development to biodiversity--Tribolium castaneum, an insect model organism for short germband development.Dev Genes Evol. 2008 Apr;218(3-4):119-26. doi: 10.1007/s00427-008-0214-3. Epub 2008 Apr 8. Dev Genes Evol. 2008. PMID: 18392874 Review.
-
Insect serosa: a head line in comparative developmental genetics.Curr Biol. 2005 Apr 12;15(7):R245-7. doi: 10.1016/j.cub.2005.03.022. Curr Biol. 2005. PMID: 15823522 Review.
Cited by
-
Sox enters the picture.Elife. 2018 Oct 1;7:e41136. doi: 10.7554/eLife.41136. Elife. 2018. PMID: 30273121 Free PMC article.
-
A damped oscillator imposes temporal order on posterior gap gene expression in Drosophila.PLoS Biol. 2018 Feb 16;16(2):e2003174. doi: 10.1371/journal.pbio.2003174. eCollection 2018 Feb. PLoS Biol. 2018. PMID: 29451884 Free PMC article.
-
Growth zone segmentation in the milkweed bug Oncopeltus fasciatus sheds light on the evolution of insect segmentation.BMC Evol Biol. 2018 Nov 28;18(1):178. doi: 10.1186/s12862-018-1293-z. BMC Evol Biol. 2018. PMID: 30486779 Free PMC article.
-
The red flour beetle T. castaneum: elaborate genetic toolkit and unbiased large scale RNAi screening to study insect biology and evolution.Evodevo. 2022 Jul 19;13(1):14. doi: 10.1186/s13227-022-00201-9. Evodevo. 2022. PMID: 35854352 Free PMC article. Review.
-
Anterior-posterior patterning of segments in Anopheles stephensi offers insights into the transition from sequential to simultaneous segmentation in holometabolous insects.J Exp Zool B Mol Dev Evol. 2023 Mar;340(2):116-130. doi: 10.1002/jez.b.23102. Epub 2021 Nov 3. J Exp Zool B Mol Dev Evol. 2023. PMID: 34734470 Free PMC article.
References
-
- Palmeirim I, Henrique D, Ish-Horowicz D, Pourquié O. Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell. 1997;91:639–648. - PubMed
-
- Dequéant ML, et al. A complex oscillating network of signaling genes underlies the mouse segmentation clock. Science. 2006;314:1595–1598. - PubMed
-
- Li Y, Fenger U, Niehrs C, Pollet N. Cyclic expression of esr9 gene in Xenopus presomitic mesoderm. Differentiation. 2003;71:83–89. - PubMed
-
- Sarrazin AF, Peel AD, Averof M. A segmentation clock with two-segment periodicity in insects. Science. 2012;336:338–341. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

