Root-associated fungal microbiota of nonmycorrhizal Arabis alpina and its contribution to plant phosphorus nutrition
- PMID: 28973917
- PMCID: PMC5676915
- DOI: 10.1073/pnas.1710455114
Root-associated fungal microbiota of nonmycorrhizal Arabis alpina and its contribution to plant phosphorus nutrition
Abstract
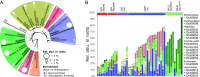
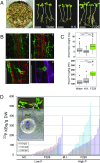
Most land plants live in association with arbuscular mycorrhizal (AM) fungi and rely on this symbiosis to scavenge phosphorus (P) from soil. The ability to establish this partnership has been lost in some plant lineages like the Brassicaceae, which raises the question of what alternative nutrition strategies such plants have to grow in P-impoverished soils. To understand the contribution of plant-microbiota interactions, we studied the root-associated fungal microbiome of Arabis alpina (Brassicaceae) with the hypothesis that some of its components can promote plant P acquisition. Using amplicon sequencing of the fungal internal transcribed spacer 2, we studied the root and rhizosphere fungal communities of A. alpina growing under natural and controlled conditions including low-P soils and identified a set of 15 fungal taxa consistently detected in its roots. This cohort included a Helotiales taxon exhibiting high abundance in roots of wild A. alpina growing in an extremely P-limited soil. Consequently, we isolated and subsequently reintroduced a specimen from this taxon into its native P-poor soil in which it improved plant growth and P uptake. The fungus exhibited mycorrhiza-like traits including colonization of the root endosphere and P transfer to the plant. Genome analysis revealed a link between its endophytic lifestyle and the expansion of its repertoire of carbohydrate-active enzymes. We report the discovery of a plant-fungus interaction facilitating the growth of a nonmycorrhizal plant under native P-limited conditions, thus uncovering a previously underestimated role of root fungal microbiota in P cycling.
Keywords: Brassicaceae; Helotiales; fungal endophyte; microbiome; nutrient transfer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Continuum of root-fungal symbioses for plant nutrition.Proc Natl Acad Sci U S A. 2017 Oct 31;114(44):11574-11576. doi: 10.1073/pnas.1716329114. Epub 2017 Oct 23. Proc Natl Acad Sci U S A. 2017. PMID: 29078417 Free PMC article. No abstract available.
References
-
- Bulgarelli D, et al. Revealing structure and assembly cues for Arabidopsis root-inhabiting bacterial microbiota. Nature. 2012;488:91–95. - PubMed
-
- Berendsen RL, Pieterse CMJ, Bakker PAHM. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012;17:478–486. - PubMed
-
- Porras-Alfaro A, Bayman P. Hidden fungi, emergent properties: Endophytes and microbiomes. Annu Rev Phytopathol. 2011;49:291–315. - PubMed
-
- Vandenkoornhuyse P, Quaiser A, Duhamel M, Le Van A, Dufresne A. The importance of the microbiome of the plant holobiont. New Phytol. 2015;206:1196–1206. - PubMed
-
- Field KJ, Pressel S, Duckett JG, Rimington WR, Bidartondo MI. Symbiotic options for the conquest of land. Trends Ecol Evol. 2015;30:477–486. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

