The European AntibotABE Framework Program and Its Update: Development of Innovative Botulinum Antibodies
- PMID: 28974033
- PMCID: PMC5666356
- DOI: 10.3390/toxins9100309
The European AntibotABE Framework Program and Its Update: Development of Innovative Botulinum Antibodies
Abstract
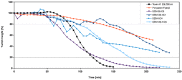
The goal of the AntiBotABE Program was the development of recombinant antibodies that neutralize botulinum neurotoxins (BoNT) A, B and E. These serotypes are lethal and responsible for most human botulinum cases. To improve therapeutic efficacy, the heavy and light chains (HC and LC) of the three BoNT serotypes were targeted to achieve a synergistic effect (oligoclonal antibodies). For antibody isolation, macaques were immunized with the recombinant and non-toxic BoNT/A, B or E, HC or LC, followed by the generation of immune phage-display libraries. Antibodies were selected from these libraries against the holotoxin and further analyzed in in vitro and ex vivo assays. For each library, the best ex vivo neutralizing antibody fragments were germline-humanized and expressed as immunoglobulin G (IgGs). The IgGs were tested in vivo, in a standardized model of protection, and challenged with toxins obtained from collections of Clostridium strains. Protective antibody combinations against BoNT/A and BoNT/B were evidenced and for BoNT/E, the anti-LC antibody alone was found highly protective. The combination of these five antibodies as an oligoclonal antibody cocktail can be clinically and regulatorily developed while their high "humanness" predicts a high tolerance in humans.
Keywords: AntiBotABE; IgG; biodefense; botulinum; botulism; neutralization; oligoclonal antibodies; phage-display; recombinant; toxin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



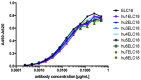
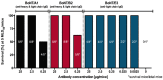

References
-
- Pelat T., Hust M., Laffly E., Condemine F., Bottex C., Vidal D., Lefranc M.-P., Dübel S., Thullier P. High-affinity, human antibody-like antibody fragment (single-chain variable fragment) neutralizing the lethal factor (LF) of Bacillus anthracis by inhibiting protective antigen-LF complex formation. Antimicrob. Agents Chemother. 2007;51:2758–2764. doi: 10.1128/AAC.01528-06. - DOI - PMC - PubMed
-
- Laffly E., Danjou L., Condemine F., Vidal D., Drouet E., Lefranc M.P., Bottex C., Thullier P. Selection of a macaque Fab with framework regions like those in humans, high affinity, and ability to neutralize the protective antigen (PA) of Bacillus anthracis by binding to the segment of PA between residues 686 and 694. Antimicrob. Agents Chemother. 2005;49:3414–3420. doi: 10.1128/AAC.49.8.3414-3420.2005. - DOI - PMC - PubMed
-
- Rülker T., Voß L., Thullier P., O’ Brien L.M., Pelat T., Perkins S.D., Langermann C., Schirrmann T., Dübel S., Marschall H.-J., et al. Isolation and characterisation of a human-like antibody fragment (scFv) that inactivates VEEV in vitro and in vivo. PLoS ONE. 2012;7:e37242. doi: 10.1371/journal.pone.0037242. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

