Using automated voice messages linked to telephone counselling to increase post-menstrual regulation contraceptive uptake and continuation in Bangladesh: study protocol for a randomised controlled trial
- PMID: 28974209
- PMCID: PMC5627401
- DOI: 10.1186/s12889-017-4703-z
Using automated voice messages linked to telephone counselling to increase post-menstrual regulation contraceptive uptake and continuation in Bangladesh: study protocol for a randomised controlled trial
Abstract
Background: Adoption of modern contraceptive methods after menstrual regulation (MR) is thought to reduce subsequent unwanted pregnancy and abortion. Long-acting reversible contraceptives (LARCs) are highly effective at reducing unintended pregnancy, but uptake in Bangladesh is low. Providing information on the most effective methods of contraception increases uptake of more effective methods. This protocol describes a randomised controlled trial of an intervention delivered by mobile phone designed to support post-MR contraceptive use in Bangladesh.
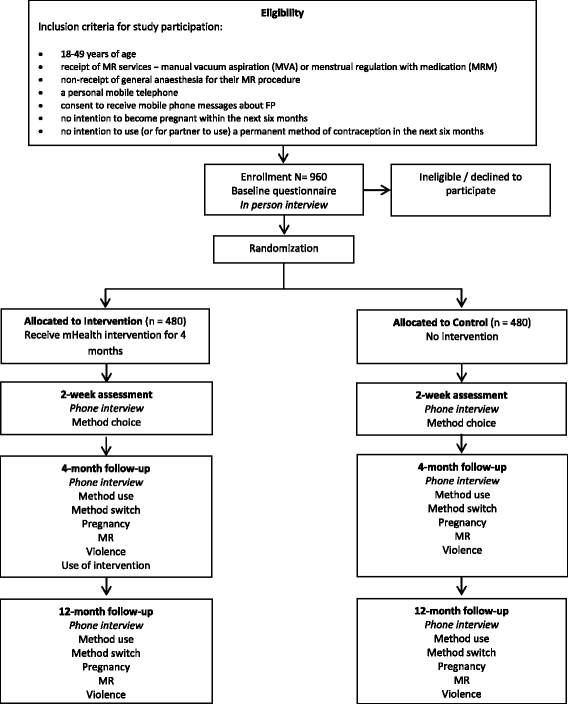
Methods: This is a multi-site single blind individual randomised controlled trial. At least 960 women undergoing MR procedures at selected facilities will be recruited after their procedure by female research assistants. Women will be randomised into the control or intervention group with a 1:1 ratio. All participants will receive usual clinic care, including contraceptive counselling and the telephone number of a non-toll-free call centre which provides counselling on MR and contraception. During the 4 months after their MR procedure, intervention participants will be sent 11 recorded interactive voice messages to their mobile phone about contraception with a focus on their chosen method and LARCs. Each message allows the participant to connect directly to the call centre. The intervention is free to the user. The control group will receive no messages delivered by mobile phone. All participants will be asked to complete an in-person questionnaire at recruitment and follow-up questionnaires by telephone at 2 weeks, 4 months and 12 months after their MR. The primary outcome for the trial will be self-reported LARC use 4 months post-MR. Secondary outcomes include LARC use at 2 weeks and 12 months post-MR, use of any effective modern contraceptive method at 2 weeks, 4 months and 12 months post-MR, and contraceptive discontinuation, contraceptive method switching, pregnancy, subsequent MR and experience of violence during the 12 month study period.
Discussion: Mobile phones offer a low-cost mechanism for providing individualised support to women with contraception outside of the clinic setting. This study will provide information on the effects of such an intervention among MR clients in Bangladesh.
Trial registration: Trial registered with clinicaltrials.gov Registration number: NCT02579785 Date of registration: 16th October 2015.
Keywords: Abortion; Bangladesh; Contraception; Family planning; Mobile phone; Post-menstrual regulation; Protocol; Randomised controlled trial; mHealth.
Conflict of interest statement
Ethics approval and consent to participate
The study has received ethical approval from the Bangladesh Medical Research Council, the London School of Hygiene and Tropical Medicine Research Ethics Committee, the Marie Stopes International Ethical Review Committee and the Population Council Institutional Review Board. Any substantial or minor amendments will be sent to the ethics committees for approval. To participate, individuals will be required to sign or thumb print an informed consent form. Consent forms will also be signed by the interviewer and by a witness chosen by the client as per the Bangladesh Medical Research Council requirements.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Government of the People’s Republic of Bangladesh Directorate General of Family Planning. Minutes of 63rd meeting of the National Technical Meeting. Dhaka, Bangladesh: 2014.
-
- Hena IA, Rob U, Sultana N, Hossain I, Yasmin R, Das TR, et al. Introducing medical Mr in Bangladesh. Dhaka, Bangladesh: 2013.
-
- Singh S, Cabigon JVJ, Hossain A, Kamal H, Perez A, Aurora EP. Estimating the level of abortion in the Philippines and Bangladesh. Int Fam Plan Perspect. 1997;23:100–107. doi: 10.2307/2950765. - DOI
-
- Guttmacher Institute. Menstrual regulation and induced abortion in Bangladesh 2012. https://www.guttmacher.org/sites/default/files/pdfs/pubs/FB-Bangladesh-M....
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous