Clinical outcomes in idursulfase-treated patients with mucopolysaccharidosis type II: 3-year data from the hunter outcome survey (HOS)
- PMID: 28974237
- PMCID: PMC5627440
- DOI: 10.1186/s13023-017-0712-3
Clinical outcomes in idursulfase-treated patients with mucopolysaccharidosis type II: 3-year data from the hunter outcome survey (HOS)
Abstract
Background: Mucopolysaccharidosis type II (MPS II; Hunter syndrome) is a rare, X-linked disorder caused by deficient activity of the enzyme iduronate-2-sulfatase (I2S). Treatment is available in the form of enzyme replacement therapy (ERT) with recombinant I2S. Clinical outcomes following ≥3 years of ERT with idursulfase were investigated in a broad population of patients with MPS II enrolled in the Hunter Outcome Survey (HOS).
Methods: As of January 2016, 639 patients (excluding female patients, individuals who had received a bone marrow transplant and those enrolled in the phase 1/2 [TKT018] or phase 2/3 [TKT024] clinical trial) followed prospectively in the registry had received idursulfase for ≥6 months. These individuals all had data available for ≥1 clinical parameter at baseline and ≥1 additional time point following treatment initiation. Changes in clinical parameters were assessed in the subcohorts of patients with a measurement at baseline and at year 1, 2 or 3 of treatment. Safety data from patients who started treatment at or after enrollment in HOS (n = 233) were also assessed.
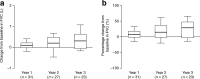
Results: Median (10th, 90th percentiles) age at first treatment was 6.2 (2.1, 18.2) years and median treatment duration was 56.3 (18.2, 97.6) months. Urinary glycosaminoglycan (uGAG) levels decreased from baseline to year 3 in patients with data available at this time point (median change from baseline: -201.0 [-591.4, -21.9] μg/mg creatinine [n = 121]). Improvements in the following parameters were observed at year 3 in the subcohorts: 6-min walking test (6MWT) distance, 10.6 (-33.6, 50.8)% (n = 26); left ventricular mass index (LVMI), -9.3 (-31.5, 19.7)% (n = 52); absolute forced vital capacity (FVC), 29.7 (-13.4, 66.7)% (n = 23); absolute forced expiratory volume in 1 s (FEV1), 22.8 (-15.2, 62.1)% (n = 22); palpable liver size, -54.5 (-85.7, 50.0)% (n = 53); palpable spleen size, -33.3 (-80.0, 33.3)% (n = 17). No new or unexpected safety concerns were identified in this analysis.
Conclusions: These findings suggest that idursulfase has a positive effect on uGAG levels, 6MWT results, LVMI, FVC, FEV1 and hepatosplenomegaly after 1, 2 and 3 years treatment.
Keywords: Disease registry; Efficacy; Hunter syndrome; Idursulfase; Lysosomal storage disease.
Conflict of interest statement
JM is a consultant to BioMarin, Eloxx, Sanofi Genzyme, Green Cross, Janssen Pharmaceuticals, PTC Therapeutics and Shire. He also serves on advisory boards for BioMarin, Sanofi Genzyme and Shire. He is principal investigator for phase 1/2 and phase 2/3 trials, sponsored by Shire, that investigate intrathecal ERT for patients with the severe form of Hunter syndrome.
RG has received travel grants from BioMarin, Sanofi Genzyme and Shire, research grants from Actelion Pharmaceuticals, Alexion, Amicus, BioMarin, Sanofi Genzyme and Shire, and honoraria for speaking engagements from Actelion Pharmaceuticals, Alexion, Amicus, BioMarin, Sanofi Genzyme, PTC Therapeutics and Shire.
MS has received grants, travel support and honoraria from Actelion Pharmaceuticals, Alexion, BioMarin, Sanofi Genzyme, Shire and PTC Therapeutics.
AT-S has received travel grants and speaker fees from BioMarin, Sanofi Genzyme, Shire and Synageva BioPharma.
Ms. Jego is an employee of Cytel, Inc. and has received consulting fees from Shire.
MB has received honoraria, travel support and unrestricted grants from Actelion Pharmaceuticals, BioMarin, Sanofi Genzyme and Shire.
Figures





Similar articles
-
Evaluation of the long-term treatment effects of intravenous idursulfase in patients with mucopolysaccharidosis II (MPS II) using statistical modeling: data from the Hunter Outcome Survey (HOS).Orphanet J Rare Dis. 2021 Oct 30;16(1):456. doi: 10.1186/s13023-021-02052-4. Orphanet J Rare Dis. 2021. PMID: 34717704 Free PMC article.
-
First experience of enzyme replacement therapy with idursulfase in Spanish patients with Hunter syndrome under 5 years of age: case observations from the Hunter Outcome Survey (HOS).Eur J Med Genet. 2010 Nov-Dec;53(6):371-7. doi: 10.1016/j.ejmg.2010.07.013. Epub 2010 Aug 10. Eur J Med Genet. 2010. PMID: 20709629
-
The relationship between anti-idursulfase antibody status and safety and efficacy outcomes in attenuated mucopolysaccharidosis II patients aged 5 years and older treated with intravenous idursulfase.Mol Genet Metab. 2013 Nov;110(3):303-10. doi: 10.1016/j.ymgme.2013.08.002. Epub 2013 Aug 9. Mol Genet Metab. 2013. PMID: 23988379 Clinical Trial.
-
A review of the clinical outcomes in idursulfase-treated and untreated Filipino patients with mucopolysaccharidosis type II: data from the local lysosomal storage disease registry.Orphanet J Rare Dis. 2021 Jul 21;16(1):323. doi: 10.1186/s13023-021-01875-5. Orphanet J Rare Dis. 2021. PMID: 34289859 Free PMC article. Review.
-
Development of idursulfase therapy for mucopolysaccharidosis type II (Hunter syndrome): the past, the present and the future.Drug Des Devel Ther. 2017 Aug 23;11:2467-2480. doi: 10.2147/DDDT.S139601. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28860717 Free PMC article. Review.
Cited by
-
Airway Findings in Patients with Hunter Syndrome Treated with Intravenous Idursulfase.J Clin Med. 2023 Jan 6;12(2):480. doi: 10.3390/jcm12020480. J Clin Med. 2023. PMID: 36675409 Free PMC article.
-
Reliability and Validity of the 6-Minute Walk Test in Hypophosphatasia.JBMR Plus. 2019 Mar 1;3(6):e10131. doi: 10.1002/jbm4.10131. eCollection 2019 Jun. JBMR Plus. 2019. PMID: 31346563 Free PMC article.
-
Intrathecal idursulfase-IT in patients with neuronopathic mucopolysaccharidosis II: Results from a phase 2/3 randomized study.Mol Genet Metab. 2022 Sep-Oct;137(1-2):127-139. doi: 10.1016/j.ymgme.2022.07.017. Epub 2022 Aug 2. Mol Genet Metab. 2022. PMID: 36027721 Free PMC article. Clinical Trial.
-
Analysis of cognitive ability and adaptive behavior assessment tools used in an observational study of patients with mucopolysaccharidosis II.Orphanet J Rare Dis. 2021 Dec 4;16(1):501. doi: 10.1186/s13023-021-02118-3. Orphanet J Rare Dis. 2021. PMID: 34863240 Free PMC article.
-
The Value of Case Reports in Systematic Reviews from Rare Diseases. The Example of Enzyme Replacement Therapy (ERT) in Patients with Mucopolysaccharidosis Type II (MPS-II).Int J Environ Res Public Health. 2020 Sep 10;17(18):6590. doi: 10.3390/ijerph17186590. Int J Environ Res Public Health. 2020. PMID: 32927819 Free PMC article.
References
-
- Neufeld EF, Muenzer J, et al. The Mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, et al., editors. The Metabolic and Molecular Bases of Inherited Disease. 8. New York: McGraw-Hill; 2001. pp. 3421–3452.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

