An intervention delivered by text message to increase the acceptability of effective contraception among young women in Palestine: study protocol for a randomised controlled trial
- PMID: 28974258
- PMCID: PMC5627444
- DOI: 10.1186/s13063-017-2191-1
An intervention delivered by text message to increase the acceptability of effective contraception among young women in Palestine: study protocol for a randomised controlled trial
Abstract
Background: Unintended pregnancy can negatively impact women's lives and is associated with poorer health outcomes for women and children. Many women, particularly in low- and middle-income countries, continue to face obstacles in avoiding unintended pregnancy. In the State of Palestine, a survey conducted in 2006 estimated that 38% of pregnancies are unintended. In 2014, unmet need for contraception was highest among young women aged 20-24 years, at 15%. Mobile phones are increasingly being used to deliver health support. Once developed, interventions delivered by mobile phone are often cheaper to deliver than face-to-face support. The London School of Hygiene and Tropical Medicine and the Palestinian Family Planning and Protection Association have partnered to develop and evaluate a contraceptive behavioural intervention for young women in Palestine delivered by mobile phone. The intervention was developed guided by behavioural science and consists of short, mobile phone text messages that contain information about contraception and behaviour change methods delivered over 4 months.
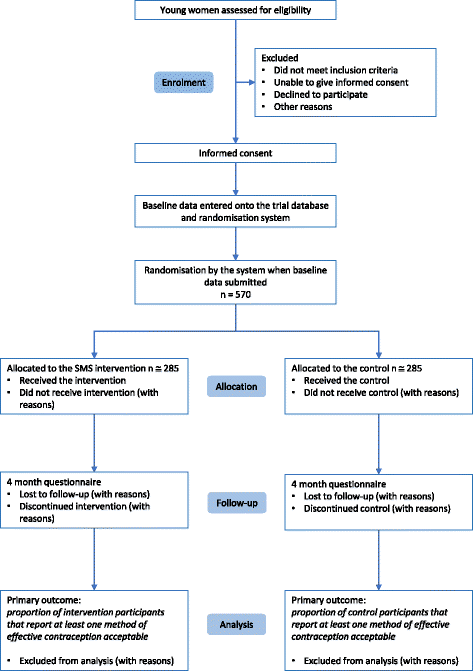
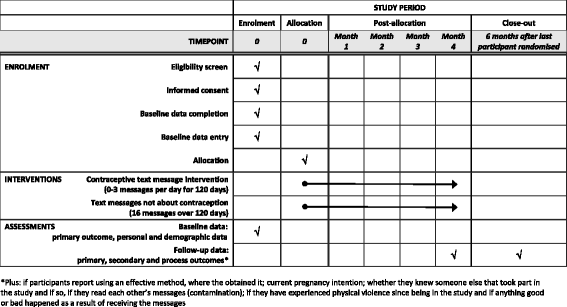
Methods: We will evaluate the intervention by conducting a randomised controlled trial. Five hundred and seventy women aged 18-24 years, who do not report using an effective method of contraception, will be allocated with a 1:1 ratio to receive the intervention text messages or control text messages about trial participation. The primary outcome is self-reported acceptability of at least one method of effective contraception at 4 months. Secondary outcomes include the use of effective contraception, acceptability of individual methods, discontinuation, service uptake, unintended pregnancy and abortion. Process outcomes include knowledge, perceived norms, personal agency and intervention dose received. Outcomes at 4 months will be compared between arms using logistic regression.
Discussion: This trial will determine the effect of the intervention on young women's attitudes towards the most effective methods of contraception. If the intervention is found to be effective, the intervention will be implemented widely across Palestine. The results could also be used to design a larger trial to establish its effect on unintended pregnancy.
Trial registration: ClinicalTrials.gov, ID: NCT02905461 . Registered on 14 September 2016.
Keywords: Cell phone; Mobile phone; Palestine. Contraception; Reproductive health; Young adults.
Conflict of interest statement
Ethics approval and consent to participate
The trial was granted ethical approval by the LSHTM Interventions Research Ethics Committee on 16 May 2016 and by the State of Palestine Ministry of Health Primary Health Care and Public Health Directorate on 9 May 2016.
Informed consent will be taken either by PFPPA recruitment staff or participants can read the Information Sheet (see Additional file 3) and provide informed consent (see Additional file 4) themselves online through the system.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Hardee K, Eggleston E, Wong EL, Irwanto, Hull TH. Unintended pregnancy and women’s psychological well-being in Indonesia. J Biosoc Sci. 2004;36(5):617–26. https://www.ncbi.nlm.nih.gov/pubmed/?term=Irwanto%5BAuthor%5D&cauthor=tr.... - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

