Improving Assessment of Drug Safety Through Proteomics: Early Detection and Mechanistic Characterization of the Unforeseen Harmful Effects of Torcetrapib
- PMID: 28974520
- PMCID: PMC5839936
- DOI: 10.1161/CIRCULATIONAHA.117.028213
Improving Assessment of Drug Safety Through Proteomics: Early Detection and Mechanistic Characterization of the Unforeseen Harmful Effects of Torcetrapib
Abstract
Background: Early detection of adverse effects of novel therapies and understanding of their mechanisms could improve the safety and efficiency of drug development. We have retrospectively applied large-scale proteomics to blood samples from ILLUMINATE (Investigation of Lipid Level Management to Understand its Impact in Atherosclerotic Events), a trial of torcetrapib (a cholesterol ester transfer protein inhibitor), that involved 15 067 participants at high cardiovascular risk. ILLUMINATE was terminated at a median of 550 days because of significant absolute increases of 1.2% in cardiovascular events and 0.4% in mortality with torcetrapib. The aims of our analysis were to determine whether a proteomic analysis might reveal biological mechanisms responsible for these harmful effects and whether harmful effects of torcetrapib could have been detected early in the ILLUMINATE trial with proteomics.
Methods: A nested case-control analysis of paired plasma samples at baseline and at 3 months was performed in 249 participants assigned to torcetrapib plus atorvastatin and 223 participants assigned to atorvastatin only. Within each treatment arm, cases with events were matched to controls 1:1. Main outcomes were a survey of 1129 proteins for discovery of biological pathways altered by torcetrapib and a 9-protein risk score validated to predict myocardial infarction, stroke, heart failure, or death.
Results: Plasma concentrations of 200 proteins changed significantly with torcetrapib. Their pathway analysis revealed unexpected and widespread changes in immune and inflammatory functions, as well as changes in endocrine systems, including in aldosterone function and glycemic control. At baseline, 9-protein risk scores were similar in the 2 treatment arms and higher in participants with subsequent events. At 3 months, the absolute 9-protein derived risk increased in the torcetrapib plus atorvastatin arm compared with the atorvastatin-only arm by 1.08% (P=0.0004). Thirty-seven proteins changed in the direction of increased risk of 49 proteins previously associated with cardiovascular and mortality risk.
Conclusions: Heretofore unknown effects of torcetrapib were revealed in immune and inflammatory functions. A protein-based risk score predicted harm from torcetrapib within just 3 months. A protein-based risk assessment embedded within a large proteomic survey may prove to be useful in the evaluation of therapies to prevent harm to patients.
Clinical trial registration: URL: https://www.clinicaltrials.gov. Unique identifier: NCT00134264.
Keywords: aptamers; biomarkers; peptides; precision medicine; prescription drugs; proteins; proteomics.
© 2017 The Authors.
Figures

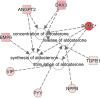
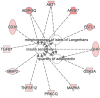
 ), transmembrane receptor (
), transmembrane receptor ( ), transporter (
), transporter ( ), and other (○). ADIPO1 indicates adiponectin; C1Q, and collagen domain containing; APOE, apolipoprotein E, FSTL3, follistatin-like 3; GHR, growth hormone receptor; MAPK8, mitogen-activated protein kinase-8; MMP2, matrix metalloproteinase-2; PRKCQ, protein kinase Cθ type; TGFB1, transforming growth factor-β1; and TNSFSF12, tumor necrosis factor superfamily member 12. *Given protein was represented in the modified aptamer assay more than once.
), and other (○). ADIPO1 indicates adiponectin; C1Q, and collagen domain containing; APOE, apolipoprotein E, FSTL3, follistatin-like 3; GHR, growth hormone receptor; MAPK8, mitogen-activated protein kinase-8; MMP2, matrix metalloproteinase-2; PRKCQ, protein kinase Cθ type; TGFB1, transforming growth factor-β1; and TNSFSF12, tumor necrosis factor superfamily member 12. *Given protein was represented in the modified aptamer assay more than once.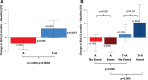
Comment in
-
Harnessing the Power of Proteomics to Assess Drug Safety and Guide Clinical Trials.Circulation. 2018 Mar 6;137(10):1011-1014. doi: 10.1161/CIRCULATIONAHA.117.032876. Circulation. 2018. PMID: 29506994 Free PMC article.
References
-
- Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B ILLUMINATE Investigators. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. - PubMed
-
- Tall AR, Yvan-Charvet L, Wang N. The failure of torcetrapib: was it the molecule or the mechanism? Arterioscler Thromb Vasc Biol. 2007;27:257–260. doi: 10.1161/01.ATV.0000256728.60226.77. - PubMed
-
- Berenson A. End of drug trial is a big loser for Pfizer. New York Times. 2006 http://www.nytimes.com/2006/12/04/health/04pfizer.html. Accessed November 28, 2016.
-
- Everett BM, Smith RJ, Hiatt WR. Reducing LDL with PCSK9 inhibitors: the clinical benefit of lipid drugs. N Engl J Med. 2015;373:1588–1591. doi: 10.1056/NEJMp1508120. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

