Modulation of Navitoclax Sensitivity by Dihydroartemisinin-Mediated MCL-1 Repression in BCR-ABL+ B-Lineage Acute Lymphoblastic Leukemia
- PMID: 28974549
- PMCID: PMC5786379
- DOI: 10.1158/1078-0432.CCR-17-1231
Modulation of Navitoclax Sensitivity by Dihydroartemisinin-Mediated MCL-1 Repression in BCR-ABL+ B-Lineage Acute Lymphoblastic Leukemia
Abstract
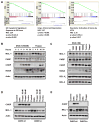
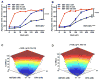
Purpose: BCR-ABL+ B-ALL leukemic cells are highly dependent on the expression of endogenous antiapoptotic MCL-1 to promote viability and are resistant to BH3-mimetic agents such as navitoclax (ABT-263) that target BCL-2, BCL-XL, and BCL-W. However, the survival of most normal blood cells and other cell types is also dependent on Mcl-1 Despite the requirement for MCL-1 in these cell types, initial reports of MCL-1-specific BH3-mimetics have not described any overt toxicities associated with single-agent use, but these agents are still early in clinical development. Therefore, we sought to identify approved drugs that could sensitize leukemic cells to ABT-263.Experimental Design: A screen identified dihydroartemisinin (DHA), a water-soluble metabolite of the antimalarial artemisinin. Using mouse and human leukemic cell lines, and primary patient-derived xenografts, the effect of DHA on survival was tested, and mechanistic studies were carried out to discover how DHA functions. We further tested in vitro and in vivo whether combining DHA with ABT-263 could enhance the response of leukemic cells to combination therapy.Results: DHA causes the downmodulation of MCL-1 expression by triggering a cellular stress response that represses translation. The repression of MCL-1 renders leukemic cells highly sensitive to synergistic cell death induced by ABT-263 in a mouse model of BCR-ABL+ B-ALL both in vitro and in vivo Furthermore, DHA synergizes with ABT-263 in human Ph+ ALL cell lines, and primary patient-derived xenografts of Ph+ ALL in culture.Conclusions: Our findings suggest that combining DHA with ABT-263 can improve therapeutic response in BCR-ABL+ B-ALL. Clin Cancer Res; 23(24); 7558-68. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Pui CH, Jeha S. New therapeutic strategies for the treatment of acute lymphoblastic leukaemia. Nat Rev Drug Discov. 2007;6(2):149–65. - PubMed
-
- Burmeister T, Schwartz S, Bartram CR, Gokbuget N, Hoelzer D, Thiel E. Patients’ age and BCR-ABL frequency in adult B-precursor ALL: a retrospective analysis from the GMALL study group. Blood. 2008;112(3):918–9. - PubMed
-
- Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243(5405):290–3. - PubMed
-
- Witte ON, Dasgupta A, Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980;283(5750):826–31. - PubMed
-
- Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105(7):2640–53. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

