Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy])
- PMID: 28974553
- PMCID: PMC6372053
- DOI: 10.1161/CIRCRESAHA.117.310712
Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients With Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy])
Abstract
Rationale: Umbilical cord-derived mesenchymal stem cells (UC-MSC) are easily accessible and expanded in vitro, possess distinct properties, and improve myocardial remodeling and function in experimental models of cardiovascular disease. Although bone marrow-derived mesenchymal stem cells have been previously assessed for their therapeutic potential in individuals with heart failure and reduced ejection fraction, no clinical trial has evaluated intravenous infusion of UC-MSCs in these patients.
Objective: Evaluate the safety and efficacy of the intravenous infusion of UC-MSC in patients with chronic stable heart failure and reduced ejection fraction.
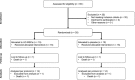
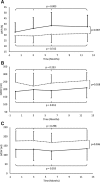
Methods and results: Patients with heart failure and reduced ejection fraction under optimal medical treatment were randomized to intravenous infusion of allogenic UC-MSCs (Cellistem, Cells for Cells S.A., Santiago, Chile; 1×106 cells/kg) or placebo (n=15 per group). UC-MSCs in vitro, compared with bone marrow-derived mesenchymal stem cells, displayed a 55-fold increase in the expression of hepatocyte growth factor, known to be involved in myogenesis, cell migration, and immunoregulation. UC-MSC-treated patients presented no adverse events related to the cell infusion, and none of the patients tested at 0, 15, and 90 days presented alloantibodies to the UC-MSCs (n=7). Only the UC-MSC-treated group exhibited significant improvements in left ventricular ejection fraction at 3, 6, and 12 months of follow-up assessed both through transthoracic echocardiography (P=0.0167 versus baseline) and cardiac MRI (P=0.025 versus baseline). Echocardiographic left ventricular ejection fraction change from baseline to month 12 differed significantly between groups (+7.07±6.22% versus +1.85±5.60%; P=0.028). In addition, at all follow-up time points, UC-MSC-treated patients displayed improvements of New York Heart Association functional class (P=0.0167 versus baseline) and Minnesota Living with Heart Failure Questionnaire (P<0.05 versus baseline). At study completion, groups did not differ in mortality, heart failure admissions, arrhythmias, or incident malignancy.
Conclusions: Intravenous infusion of UC-MSC was safe in this group of patients with stable heart failure and reduced ejection fraction under optimal medical treatment. Improvements in left ventricular function, functional status, and quality of life were observed in patients treated with UC-MSCs.
Clinical trial registration: URL: https://www.clinicaltrials.gov/ct2/show/NCT01739777. Unique identifier: NCT01739777.
Keywords: cardiomyopathies; clinical trial; heart failure; mesenchymal stromal cells; umbilical cord.
© 2017 The Authors.
Figures

Comment in
-
Cardiac Cell Therapy Evolving From Complex to Straightforward: Enabling Adoption and Affordability.Circ Res. 2017 Oct 27;121(10):1116-1118. doi: 10.1161/CIRCRESAHA.117.311947. Circ Res. 2017. PMID: 29074527 Free PMC article. No abstract available.
References
-
- Jeevanantham V, Butler M, Saad A, Abdel-Latif A, Zuba-Surma EK, Dawn B. Adult bone marrow cell therapy improves survival and induces long-term improvement in cardiac parameters: a systematic review and meta-analysis. Circulation. 2012;126:551–568. doi: 10.1161/CIRCULATIONAHA.111.086074. - PMC - PubMed
-
- Kandala J, Upadhyay GA, Pokushalov E, Wu S, Drachman DE, Singh JP. Meta-analysis of stem cell therapy in chronic ischemic cardiomyopathy. Am J Cardiol. 2013;112:217–225. doi: 10.1016/j.amjcard.2013.03.021. - PubMed
-
- Gho JM, Kummeling GJ, Koudstaal S, Jansen Of Lorkeers SJ, Doevendans PA, Asselbergs FW, Chamuleau SA. Cell therapy, a novel remedy for dilated cardiomyopathy? A systematic review. J Card Fail. 2013;19:494–502. doi: 10.1016/j.cardfail.2013.05.006. - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

