Thyroid and Glucocorticoid Hormones Promote Functional T-Tubule Development in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes
- PMID: 28974554
- PMCID: PMC5722667
- DOI: 10.1161/CIRCRESAHA.117.311920
Thyroid and Glucocorticoid Hormones Promote Functional T-Tubule Development in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes
Abstract
Rationale: Human-induced pluripotent stem cell-derived cardiomyocytes (hiPSC-CM) are increasingly being used for modeling heart disease and are under development for regeneration of the injured heart. However, incomplete structural and functional maturation of hiPSC-CM, including lack of T-tubules, immature excitation-contraction coupling, and inefficient Ca-induced Ca release remain major limitations.
Objective: Thyroid and glucocorticoid hormones are critical for heart maturation. We hypothesized that their addition to standard protocols would promote T-tubule development and mature excitation-contraction coupling of hiPSC-CM when cultured on extracellular matrix with physiological stiffness (Matrigel mattress).
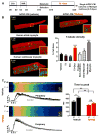
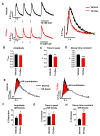
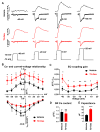
Methods and results: hiPSC-CM were generated using a standard chemical differentiation method supplemented with T3 (triiodothyronine) and/or Dex (dexamethasone) during days 16 to 30 followed by single-cell culture for 5 days on Matrigel mattress. hiPSC-CM treated with T3+Dex, but not with either T3 or Dex alone, developed an extensive T-tubule network. Notably, Matrigel mattress was necessary for T-tubule formation. Compared with adult human ventricular cardiomyocytes, T-tubules in T3+Dex-treated hiPSC-CM were less organized and had more longitudinal elements. Confocal line scans demonstrated spatially and temporally uniform Ca release that is characteristic of excitation-contraction coupling in the heart ventricle. T3+Dex enhanced elementary Ca release measured by Ca sparks and promoted RyR2 (ryanodine receptor) structural organization. Simultaneous measurements of L-type Ca current and intracellular Ca release confirmed enhanced functional coupling between L-type Ca channels and RyR2 in T3+Dex-treated cells.
Conclusions: Our results suggest a permissive role of combined thyroid and glucocorticoid hormones during the cardiac differentiation process, which when coupled with further maturation on Matrigel mattress, is sufficient for T-tubule development, enhanced Ca-induced Ca release, and more ventricular-like excitation-contraction coupling. This new hormone maturation method could advance the use of hiPSC-CM for disease modeling and cell-based therapy.
Keywords: calcium; cardiac electrophysiology; extracellular matrix; stem cells.
© 2017 American Heart Association, Inc.
Figures

Comment in
-
A Recipe for T-Tubules in Human iPS Cell-Derived Cardiomyocytes.Circ Res. 2017 Dec 8;121(12):1294-1295. doi: 10.1161/CIRCRESAHA.117.312177. Circ Res. 2017. PMID: 29217703 Free PMC article. No abstract available.
References
-
- Kolanowski TJ, Antos CL, Guan K. Making human cardiomyocytes up to date: Derivation, maturation state and perspectives. Int J Cardiol. 2017 - PubMed
-
- Snir M, Kehat I, Gepstein A, Coleman R, Itskovitz-Eldor J, Livne E, Gepstein L. Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes. Am J Physiol Heart Circ Physiol. 2003;285:H2355–2363. - PubMed
-
- Kamakura T, Makiyama T, Sasaki K, Yoshida Y, Wuriyanghai Y, Chen J, Hattori T, Ohno S, Kita T, Horie M, Yamanaka S, Kimura T. Ultrastructural maturation of human-induced pluripotent stem cell-derived cardiomyocytes in a long-term culture. Circ J. 2013;77:1307–1314. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

