Autophagy and mitochondrial biogenesis impairment contribute to age-dependent liver injury in experimental sepsis: dysregulation of AMP-activated protein kinase pathway
- PMID: 28974562
- PMCID: PMC5888394
- DOI: 10.1096/fj.201700576R
Autophagy and mitochondrial biogenesis impairment contribute to age-dependent liver injury in experimental sepsis: dysregulation of AMP-activated protein kinase pathway
Abstract
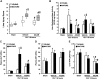
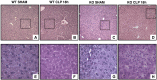
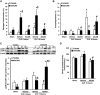
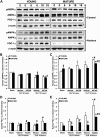
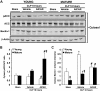
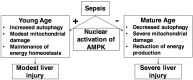
Age is an independent risk factor of multiple organ failure in patients with sepsis. However, the age-related mechanisms of injury are not known. AMPK is a crucial regulator of energy homeostasis, which controls mitochondrial biogenesis by activation of peroxisome proliferator-activated receptor-γ coactivator-α (PGC-1α) and disposal of defective organelles by autophagy. We investigated whether AMPK dysregulation might contribute to age-dependent liver injury in young (2-3 mo) and mature male mice (11-13 mo) subjected to sepsis. Liver damage was higher in mature mice than in young mice and was associated with impairment of hepatocyte mitochondrial function, structure, and biogenesis and reduced autophagy. At molecular analysis, there was a time-dependent nuclear translocation of the active phosphorylated catalytic subunits AMPKα1/α2 and PGC-1α in young, but not in mature, mice after sepsis. Treatment with the AMPK activator 5-amino-4-imidazolecarboxamide riboside-1-β-d-ribofuranoside (AICAR) improved liver mitochondrial structure in both age groups compared with vehicle. In loss-of-function studies, young knockout mice with systemic deficiency of AMPKα1 exhibited greater liver injury than did wild-type mice after sepsis. Our study suggests that AMPK is important for liver metabolic recovery during sepsis. Although its function may diminish with age, pharmacological activation of AMPK may be of therapeutic benefit.-Inata, Y., Kikuchi, S., Samraj, R. S., Hake, P. W., O'Connor, M., Ledford, J. R., O'Connor, J., Lahni, P., Wolfe, V., Piraino, G., Zingarelli, B. Autophagy and mitochondrial biogenesis impairment contribute to age-dependent liver injury in experimental sepsis: dysregulation of AMP-activated protein kinase pathway.
Keywords: AICAR; MODS; PGC-1α; cecal ligation and puncture.
Conflict of interest statement
The authors thank Dr. Benoit Viollet (INSERM and Cochin Institute, University Paris Descartes, Paris, France) for providing AMPK-α1 WT and KO mice. This work was supported by the U.S. National Institutes of Health (NIH) National Institute of General Medical Sciences Grant R01 GM-067202 (to B.Z.) and, in part, by NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant P30 DK-078392 to the Digestive Research Core Center (Integrative Morphology Core). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare no conflicts of interest.
Figures



References
-
- Singer M., Deutschman C. S., Seymour C. W., Shankar-Hari M., Annane D., Bauer M., Bellomo R., Bernard G. R., Chiche J. D., Coopersmith C. M., Hotchkiss R. S., Levy M. M., Marshall J. C., Martin G. S., Opal S. M., Rubenfeld G. D., van der Poll T., Vincent J. L., Angus D. C. (2016) The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 315, 801–810 - PMC - PubMed
-
- Angus D. C., Linde-Zwirble W. T., Lidicker J., Clermont G., Carcillo J., Pinsky M. R. (2001) Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310 - PubMed
-
- Martin G. S., Mannino D. M., Moss M. (2006) The effect of age on the development and outcome of adult sepsis. Crit. Care Med. 34, 15–21 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

