Molecular and Physiological Logics of the Pyruvate-Induced Response of a Novel Transporter in Bacillus subtilis
- PMID: 28974613
- PMCID: PMC5626966
- DOI: 10.1128/mBio.00976-17
Molecular and Physiological Logics of the Pyruvate-Induced Response of a Novel Transporter in Bacillus subtilis
Abstract
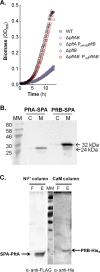
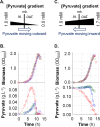
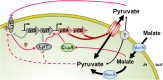
At the heart of central carbon metabolism, pyruvate is a pivotal metabolite in all living cells. Bacillus subtilis is able to excrete pyruvate as well as to use it as the sole carbon source. We herein reveal that ysbAB (renamed pftAB), the only operon specifically induced in pyruvate-grown B. subtilis cells, encodes a hetero-oligomeric membrane complex which operates as a facilitated transport system specific for pyruvate, thereby defining a novel class of transporter. We demonstrate that the LytST two-component system is responsible for the induction of pftAB in the presence of pyruvate by binding of the LytT response regulator to a palindromic region upstream of pftAB We show that both glucose and malate, the preferred carbon sources for B. subtilis, trigger the binding of CcpA upstream of pftAB, which results in its catabolite repression. However, an additional CcpA-independent mechanism represses pftAB in the presence of malate. Screening a genome-wide transposon mutant library, we find that an active malic enzyme replenishing the pyruvate pool is required for this repression. We next reveal that the higher the influx of pyruvate, the stronger the CcpA-independent repression of pftAB, which suggests that intracellular pyruvate retroinhibits pftAB induction via LytST. Such a retroinhibition challenges the rational design of novel nature-inspired sensors and synthetic switches but undoubtedly offers new possibilities for the development of integrated sensor/controller circuitry. Overall, we provide evidence for a complete system of sensors, feed-forward and feedback controllers that play a major role in environmental growth of B. subtilisIMPORTANCE Pyruvate is a small-molecule metabolite ubiquitous in living cells. Several species also use it as a carbon source as well as excrete it into the environment. The bacterial systems for pyruvate import/export have yet to be discovered. Here, we identified in the model bacterium Bacillus subtilis the first import/export system specific for pyruvate, PftAB, which defines a novel class of transporter. In this bacterium, extracellular pyruvate acts as the signal molecule for the LytST two-component system (TCS), which in turn induces expression of PftAB. However, when the pyruvate influx is high, LytST activity is drastically retroinhibited. Such a retroinhibition challenges the rational design of novel nature-inspired sensors and synthetic switches but undoubtedly offers new possibilities for the development of integrated sensor/controller circuitry.
Keywords: Bacillus subtilis; LytST; PftA PftB; YsbA YsbB; catabolite repression; malate; pyruvate transport; two-component regulatory systems.
Copyright © 2017 Charbonnier et al.
Figures



Comment in
-
The logics of metabolic regulation in bacteria challenges biosensor-based metabolic engineering.Microb Cell. 2017 Dec 11;5(1):56-59. doi: 10.15698/mic2018.01.610. Microb Cell. 2017. PMID: 29354650 Free PMC article.
References
-
- Allard-Massicotte R, Tessier L, Lécuyer F, Lakshmanan V, Lucier JF, Garneau D, Caudwell L, Vlamakis H, Bais HP, Beauregard PB. 2016. Bacillus subtilis early colonization of Arabidopsis thaliana roots involves multiple chemotaxis receptors. mBio 7:e01664-16. doi: 10.1128/mBio.01664-16. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
