An essential role for IGF2 in cartilage development and glucose metabolism during postnatal long bone growth
- PMID: 28974642
- PMCID: PMC5665487
- DOI: 10.1242/dev.155598
An essential role for IGF2 in cartilage development and glucose metabolism during postnatal long bone growth
Abstract
Postnatal bone growth involves a dramatic increase in length and girth. Intriguingly, this period of growth is independent of growth hormone and the underlying mechanism is poorly understood. Recently, an IGF2 mutation was identified in humans with early postnatal growth restriction. Here, we show that IGF2 is essential for longitudinal and appositional murine postnatal bone development, which involves proper timing of chondrocyte maturation and perichondrial cell differentiation and survival. Importantly, the Igf2 null mouse model does not represent a simple delay of growth but instead uncoordinated growth plate development. Furthermore, biochemical and two-photon imaging analyses identified elevated and imbalanced glucose metabolism in the Igf2 null mouse. Attenuation of glycolysis rescued the mutant phenotype of premature cartilage maturation, thereby indicating that IGF2 controls bone growth by regulating glucose metabolism in chondrocytes. This work links glucose metabolism with cartilage development and provides insight into the fundamental understanding of human growth abnormalities.
Keywords: Cartilage; Endochondral ossification; Glucose metabolism; Growth plate; IGF2; Postnatal.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures



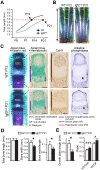
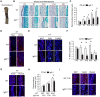
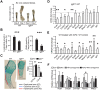

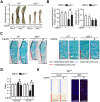

References
-
- Akiyama H., Chaboissier M. C., Martin J. F., Schedl A. and de Crombrugghe B. (2002). The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 16, 2813-2828. 10.1101/gad.1017802 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

