Maternal Gestational Diabetes Mellitus increases placental and foetal lipoprotein-associated Phospholipase A2 which might exert protective functions against oxidative stress
- PMID: 28974763
- PMCID: PMC5626711
- DOI: 10.1038/s41598-017-13051-6
Maternal Gestational Diabetes Mellitus increases placental and foetal lipoprotein-associated Phospholipase A2 which might exert protective functions against oxidative stress
Abstract
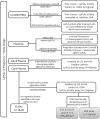
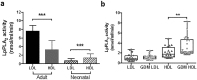
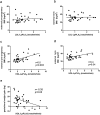
Increased Lipoprotein associated phospholipase A2 (LpPLA2) has been associated with inflammatory pathologies, including Type 2 Diabetes. Studies on LpPLA2 and Gestational Diabetes Mellitus (GDM) are rare, and have focused mostly on maternal outcome. In the present study, we investigated whether LpPLA2 activity on foetal lipoproteins is altered by maternal GDM and/or obesity (a major risk factor for GDM), thereby contributing to changes in lipoprotein functionality. We identified HDL as the major carrier of LpPLA2 activity in the foetus, which is in contrast to adults. We observed marked expression of LpPLA2 in placental macrophages (Hofbauer cells; HBCs) and found that LpPLA2 activity in these cells was increased by insulin, leptin, and pro-inflammatory cytokines. These regulators were also increased in plasma of children born from GDM pregnancies. Our results suggest that insulin, leptin, and pro-inflammatory cytokines are positive regulators of LpPLA2 activity in the foeto-placental unit. Of particular interest, functional assays using a specific LpPLA2 inhibitor suggest that high-density lipoprotein (HDL)-associated LpPLA2 exerts anti-oxidative, athero-protective functions on placental endothelium and foetus. Our results therefore raise the possibility that foetal HDL-associated LpPLA2 might act as an anti-inflammatory enzyme improving vascular barrier function.
Conflict of interest statement
The authors declare that this study was performed in the absence of any financial or commercial conflicts of interest.
Figures





Similar articles
-
Fetal High-Density Lipoproteins: Current Knowledge on Particle Metabolism, Composition and Function in Health and Disease.Biomedicines. 2021 Mar 30;9(4):349. doi: 10.3390/biomedicines9040349. Biomedicines. 2021. PMID: 33808220 Free PMC article. Review.
-
Lipoprotein-associated phospholipase A2 distribution among lipoproteins differs in type 1 diabetes.J Clin Lipidol. 2016 May-Jun;10(3):577-86. doi: 10.1016/j.jacl.2016.01.001. Epub 2016 Jan 30. J Clin Lipidol. 2016. PMID: 27206945
-
Phospholipid transfer protein in the placental endothelium is affected by gestational diabetes mellitus.J Clin Endocrinol Metab. 2012 Feb;97(2):437-45. doi: 10.1210/jc.2011-1942. Epub 2011 Nov 16. J Clin Endocrinol Metab. 2012. PMID: 22090281
-
Mitochondrial dysfunction in the fetoplacental unit in gestational diabetes mellitus.Biochim Biophys Acta Mol Basis Dis. 2020 Dec 1;1866(12):165948. doi: 10.1016/j.bbadis.2020.165948. Epub 2020 Aug 29. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 32866635 Review.
-
Expression of Lipoprotein associated phospholipase A2 enzyme in medical undergraduate students with metabolic syndrome.Diabetes Metab Syndr. 2016 Jan-Mar;10(1 Suppl 1):S21-4. doi: 10.1016/j.dsx.2015.09.003. Epub 2015 Oct 3. Diabetes Metab Syndr. 2016. PMID: 26460076
Cited by
-
Exploring the Role of Mediterranean and Westernized Diets and Their Main Nutrients in the Modulation of Oxidative Stress in the Placenta: A Narrative Review.Antioxidants (Basel). 2023 Oct 26;12(11):1918. doi: 10.3390/antiox12111918. Antioxidants (Basel). 2023. PMID: 38001771 Free PMC article. Review.
-
Maternal obesity and offspring cardiovascular remodelling - the effect of preconception and antenatal lifestyle interventions: a systematic review.Int J Obes (Lond). 2024 Aug;48(8):1045-1064. doi: 10.1038/s41366-024-01536-0. Epub 2024 Jun 19. Int J Obes (Lond). 2024. PMID: 38898228 Free PMC article.
-
Fetal High-Density Lipoproteins: Current Knowledge on Particle Metabolism, Composition and Function in Health and Disease.Biomedicines. 2021 Mar 30;9(4):349. doi: 10.3390/biomedicines9040349. Biomedicines. 2021. PMID: 33808220 Free PMC article. Review.
-
The Beneficial Effects of Alpha Lipoic Acid Supplementation on Lp-PLA2 Mass and Its Distribution between HDL and apoB-Containing Lipoproteins in Type 2 Diabetic Patients: A Randomized, Double-Blind, Placebo-Controlled Trial.Oxid Med Cell Longev. 2020 Mar 9;2020:5850865. doi: 10.1155/2020/5850865. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32256955 Free PMC article. Clinical Trial.
-
Lipoprotein-associated phospholipase A2: The story continues.Med Res Rev. 2020 Jan;40(1):79-134. doi: 10.1002/med.21597. Epub 2019 May 29. Med Res Rev. 2020. PMID: 31140638 Free PMC article. Review.
References
-
- Tarbet EB, et al. Liver cells secrete the plasma form of platelet-activating factor acetylhydrolase. J. Biol. Chem. 1991;266:16667–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

