Identification of key regions and residues controlling Aβ folding and assembly
- PMID: 28974765
- PMCID: PMC5626695
- DOI: 10.1038/s41598-017-10845-6
Identification of key regions and residues controlling Aβ folding and assembly
Abstract
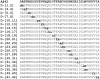
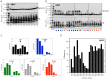
Amyloid β-protein (Aβ) assembly is hypothesized to be a seminal neuropathologic event in Alzheimer's disease (AD). We used an unbiased D-amino acid substitution strategy to determine structure-assembly relationships of 76 different Aβ40 and Aβ42 peptides. We determined the effects of the substitutions on peptide oligomerization, secondary structure dynamics, fibril assembly dynamics, and fibril morphology. Our experiments revealed that the assembly of Aβ42 was more sensitive to chiral substitutions than was Aβ40 assembly. Substitutions at identical positions in the two peptides often, but not always, produced the same effects on assembly. Sites causing substantial effects in both Aβ40 and Aβ42 include His14, Gln15, Ala30, Ile31, Met35, and Val36. Sites whose effects were unique to Aβ40 include Lys16, Leu17, and Asn 27, whereas sites unique to Aβ42 include Phe20 and Ala21. These sites may be appropriate targets for therapeutic agents that inhibit or potentiate, respectively, these effects.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures







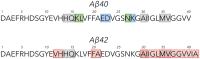
References
-
- De Strooper B. Proteases and proteolysis in Alzheimer disease: a multifactorial view on the disease process. Phys Rev. 2010;90:465–494. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

