The neurotrophic effects of different human dental mesenchymal stem cells
- PMID: 28974767
- PMCID: PMC5626751
- DOI: 10.1038/s41598-017-12969-1
The neurotrophic effects of different human dental mesenchymal stem cells
Abstract
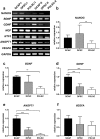
The current gold standard treatment for peripheral nerve injury is nerve grafting but this has disadvantages such as donor site morbidity. New techniques focus on replacing these grafts with nerve conduits enhanced with growth factors and/or various cell types such as mesenchymal stem cells (MSCs). Dental-MSCs (D-MSCs) including stem cells obtained from apical papilla (SCAP), dental pulp stem cells (DPSC), and periodontal ligament stem cells (PDLSC) are potential sources of MSCs for nerve repair. Here we present the characterization of various D-MSCs from the same human donors for peripheral nerve regeneration. SCAP, DPSC and PDLSC expressed BDNF, GDNF, NGF, NTF3, ANGPT1 and VEGFA growth factor transcripts. Conditioned media from D-MSCs enhanced neurite outgrowth in an in vitro assay. Application of neutralizing antibodies showed that brain derived neurotrophic factor plays an important mechanistic role by which the D-MSCs stimulate neurite outgrowth. SCAP, DPSC and PDLSC were used to treat a 10 mm nerve gap defect in a rat sciatic nerve injury model. All the stem cell types significantly enhanced axon regeneration after two weeks and showed neuroprotective effects on the dorsal root ganglia neurons. Overall the results suggested SCAP to be the optimal dental stem cell type for peripheral nerve repair.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Isaacs, J. Treatment of acute peripheral nerve injuries: current concepts. J Hand Surg Am35, 491–497 quiz 498, 10.1016/j.jhsa.2009.12.009 (2010). - PubMed
-
- Wiberg M, Terenghi G. Will it be possible to produce peripheral nerves? Surg Technol Int. 2003;11:303–310. - PubMed
-
- Aberg M, et al. Clinical evaluation of a resorbable wrap-around implant as an alternative to nerve repair: a prospective, assessor-blinded, randomised clinical study of sensory, motor and functional recovery after peripheral nerve repair. J Plast Reconstr Aesthet Surg. 2009;62:1503–1509. doi: 10.1016/j.bjps.2008.06.041. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

