AMPK: guardian of metabolism and mitochondrial homeostasis
- PMID: 28974774
- PMCID: PMC5780224
- DOI: 10.1038/nrm.2017.95
AMPK: guardian of metabolism and mitochondrial homeostasis
Abstract
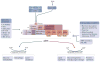
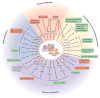
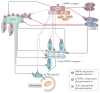
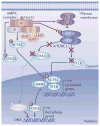
Cells constantly adapt their metabolism to meet their energy needs and respond to nutrient availability. Eukaryotes have evolved a very sophisticated system to sense low cellular ATP levels via the serine/threonine kinase AMP-activated protein kinase (AMPK) complex. Under conditions of low energy, AMPK phosphorylates specific enzymes and growth control nodes to increase ATP generation and decrease ATP consumption. In the past decade, the discovery of numerous new AMPK substrates has led to a more complete understanding of the minimal number of steps required to reprogramme cellular metabolism from anabolism to catabolism. This energy switch controls cell growth and several other cellular processes, including lipid and glucose metabolism and autophagy. Recent studies have revealed that one ancestral function of AMPK is to promote mitochondrial health, and multiple newly discovered targets of AMPK are involved in various aspects of mitochondrial homeostasis, including mitophagy. This Review discusses how AMPK functions as a central mediator of the cellular response to energetic stress and mitochondrial insults and coordinates multiple features of autophagy and mitochondrial biology.
Conflict of interest statement
The authors declare no competing interests.
Figures
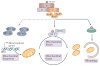
References
-
- Celenza JL, Carlson M. A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science. 1986;233:1175–1180. - PubMed
-
- Gancedo JM. Carbon catabolite repression in yeast. Eur J Biochem. 1992;206:297–313. - PubMed
-
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

