Environmental perturbation of the circadian clock during pregnancy leads to transgenerational mood disorder-like behaviors in mice
- PMID: 28974783
- PMCID: PMC5626699
- DOI: 10.1038/s41598-017-13067-y
Environmental perturbation of the circadian clock during pregnancy leads to transgenerational mood disorder-like behaviors in mice
Abstract
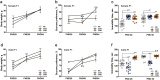
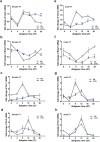
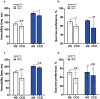
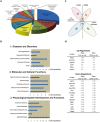
It remains unknown whether chronic circadian disturbance (CCD) during pregnancy can lead to mood disorders in the offspring. Here we show that pregnant mice in the F0 generation that were exposed to CCD stress displayed depression-like behaviors, and produced offspring in the F1 and F2 generations that also exhibited mood-associated behavioral phenotypes despite the lack of direct stressful experiences during their postnatal or adult period. Prenatal CCD stress was correlated with the elevation of plasma corticosterone levels in F1 mice. Furthermore, the diurnal expression profiles of core circadian clock genes were disrupted in the suprachiasmatic nucleus of F1 mice. Proteomics analysis revealed that prenatal CCD stress resulted in distinct changes in protein expression in the hypothalamus of female F1 mice, in particular proteins that were associated with cellular activities, metabolism, development and diseases. Sex-specific differences in melanocortin 4 receptor expression were apparent in the CCD F1 generation. We conclude that maternal exposure to chronic circadian disturbance during pregnancy can lead to sex-specific mood disorders that persist for at least two filial generations. The underlying mechanisms may depend on transgenerational changes in plasma corticosterone levels, circadian pacemaking, and hypothalamic protein expression.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

