Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer
- PMID: 28975219
- PMCID: PMC6583041
- DOI: 10.1001/jamaoncol.2017.2925
Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer
Abstract
Importance: Immune-related adverse events (irAEs) have been associated with the efficacy of PD-1 (programmed cell death protein 1) inhibitors in patients with melanoma, but whether such an association exists for non-small-cell lung cancer (NSCLC) has remained unknown.
Objective: To evaluate the relation of irAEs to nivolumab efficacy in NSCLC.
Design, setting, and participants: In this study based on landmark and multivariable analyses, a total of 134 patients with advanced or recurrent NSCLC who were treated with nivolumab in the second-line setting or later between December 2015 and August 2016 were identified from a review of medical records from multiple institutions, including a university hospital and community hospitals. Data were updated as of December 31, 2016.
Exposures: The absence or presence of any irAE before the landmark date.
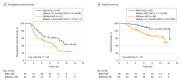
Main outcomes and measures: Kaplan-Meier curves of progression-free survival (PFS) according to the development of irAEs in 6-week landmark analysis were evaluated with the log-rank test as a preplanned primary objective. Overall survival (OS) was similarly evaluated. Multivariable analysis of both PFS and OS was performed with Cox proportional hazard regression models.
Results: In a cohort of 134 patients (median [range] age, 68 [33-85] years; 90 men [67%], 44 women [33%]), irAEs were observed in 69 of the 134 study patients (51%), including 12 patients (9%) with such events of grade 3 or 4, and 24 patients (18%) requiring systemic corticosteroid therapy. In 6-week landmark analysis, median PFS was 9.2 months (95% CI, 4.4 to not reached [NR]) and 4.8 months (95% CI, 3.0 to 7.5) (P = .04) whereas median OS was NR (95% CI, 12.3 to NR) and 11.1 months (95% CI, 9.6 to NR) (P = .01) for patients with or without irAEs, respectively. Multivariable analysis also revealed that irAEs were positively associated with survival outcome, with hazard ratios of 0.525 (95% CI, 0.287 to 0.937; P = .03) for PFS and 0.282 (95% CI, 0.101 to 0.667; P = .003) for OS.
Conclusions and relevance: Development of irAEs was associated with survival outcome of nivolumab treatment in patients with advanced or recurrent NSCLC. Further studies are needed to confirm our findings.
Conflict of interest statement
Figures
Comment in
-
Immune-Related Adverse Events-A Novel Prediction of Nivolumab Efficacy?-Reply.JAMA Oncol. 2018 Jul 1;4(7):1017-1018. doi: 10.1001/jamaoncol.2018.0751. JAMA Oncol. 2018. PMID: 29852041 No abstract available.
-
Immune-Related Adverse Events-A Novel Prediction of Nivolumab Efficacy?JAMA Oncol. 2018 Jul 1;4(7):1017. doi: 10.1001/jamaoncol.2018.0745. JAMA Oncol. 2018. PMID: 29852042 No abstract available.
References
-
- Herbst RS, Baas P, Kim D-W, et al. . Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550. - PubMed
-
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. ; KEYNOTE-024 Investigators . Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. - PubMed
-
- Boutros C, Tarhini A, Routier E, et al. . Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat Rev Clin Oncol. 2016;13(8):473-486. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials