Association of Primary Tumor Site With Mortality in Patients Receiving Bevacizumab and Cetuximab for Metastatic Colorectal Cancer
- PMID: 28975237
- PMCID: PMC5833618
- DOI: 10.1001/jamasurg.2017.3466
Association of Primary Tumor Site With Mortality in Patients Receiving Bevacizumab and Cetuximab for Metastatic Colorectal Cancer
Abstract
Importance: Biologic therapy (BT) (eg, bevacizumab or cetuximab) is increasingly used to treat metastatic colorectal cancer (mCRC). Recent investigations have suggested that right- or left-sided primary tumor origin affects survival and response to BT.
Objective: To evaluate the association of tumor origin with mortality in a diverse population-based data set of patients receiving systemic chemotherapy (SC) and bevacizumab or cetuximab for mCRC.
Design, setting, and participants: This population-based nonconcurrent cohort study of statewide California Cancer Registry data included all patients aged 40 to 85 years diagnosed with mCRC and treated with SC only or SC plus bevacizumab or cetuximab from January 1, 2004, through December 31, 2014. Patients were stratified by tumor origin in the left vs right sides.
Interventions: Treatment with SC or SC plus bevacizumab or cetuximab.
Main outcomes and measures: Mortality hazards by tumor origin (right vs left sides) were assessed for patients receiving SC alone or SC plus bevacizumab or cetuximab. Subgroup analysis for patients with wild-type KRAS tumors was also performed.
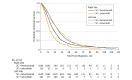
Results: A total of 11 905 patients with mCRC (6713 men [56.4%] and 5192 women [43.6%]; mean [SD] age, 60.0 [10.9] years) were eligible for the study. Among these, 4632 patients received SC and BT. Compared with SC alone, SC plus bevacizumab reduced mortality among patients with right- and left-sided mCRC, whereas SC plus cetuximab reduced mortality only among patients with left-sided tumors and was associated with significantly higher mortality for right-sided tumors (hazard ratio [HR], 1.31; 95% CI, 1.14-1.51; P < .001). Among patients treated with SC plus BT, right-sided tumor origin was associated with higher mortality among patients receiving bevacizumab (HR, 1.31; 95% CI, 1.25-1.36; P < .001) and cetuximab (HR, 1.88; 95% CI, 1.68-2.12; P < .001) BT, compared with left-sided tumor origin. In patients with wild-type KRAS tumors (n = 668), cetuximab was associated with reduced mortality among only patients with left-sided mCRC compared with bevacizumab (HR, 0.75; 95% CI, 0.63-0.90; P = .002), whereas patients with right-sided mCRC had more than double the mortality compared with those with left-sided mCRC (HR, 2.44; 95% CI, 1.83-3.25, P < .001).
Conclusions and relevance: Primary tumor site is associated with response to BT in mCRC. Right-sided primary tumor location is associated with higher mortality regardless of BT type. In patients with wild-type KRAS tumors, treatment with cetuximab benefited only those with left-sided mCRC and was associated with significantly poorer survival among those with right-sided mCRC. Our results underscore the importance of stratification by tumor site for current treatment guidelines and future clinical trials.
Conflict of interest statement
Figures

Comment in
-
Progress in the March to Precision Cancer Medicine: Left, Right, Left.JAMA Surg. 2018 Jan 1;153(1):67-68. doi: 10.1001/jamasurg.2017.3895. JAMA Surg. 2018. PMID: 28975234 No abstract available.
References
-
- Bullard Dunn KM, Rothenberger DA. Colon, rectum, and anus In: Brunicardi FC, Andersen DK, Billiar TR, et al. , eds. Schwartz’s Principles of Surgery. 10th ed. New York, NY: McGraw-Hill Education; 2014.
-
- Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113(10):779-788. - PubMed
-
- Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101(5):403-408. - PubMed
-
- Missiaglia E, Jacobs B, D’Ario G, et al. Distal and proximal colon cancers differ in terms of molecular, pathological, and clinical features. Ann Oncol. 2014;25(10):1995-2001. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

