Targeting Pioglitazone Hydrochloride Therapy After Stroke or Transient Ischemic Attack According to Pretreatment Risk for Stroke or Myocardial Infarction
- PMID: 28975241
- PMCID: PMC5710663
- DOI: 10.1001/jamaneurol.2017.2136
Targeting Pioglitazone Hydrochloride Therapy After Stroke or Transient Ischemic Attack According to Pretreatment Risk for Stroke or Myocardial Infarction
Abstract
Importance: There is growing recognition that patients may respond differently to therapy and that the average treatment effect from a clinical trial may not apply equally to all candidates for a therapy.
Objective: To determine whether, among patients with an ischemic stroke or transient ischemic attack and insulin resistance, those at higher risk for future stroke or myocardial infarction (MI) derive more benefit from the insulin-sensitizing drug pioglitazone hydrochloride compared with patients at lower risk.
Design, setting, and participants: A secondary analysis was conducted of the Insulin Resistance Intervention After Stroke trial, a double-blind, placebo-controlled trial of pioglitazone for secondary prevention. Patients were enrolled from 179 research sites in 7 countries from February 7, 2005, to January 15, 2013, and were followed up for a mean of 4.1 years through the study's end on July 28, 2015. Eligible participants had a qualifying ischemic stroke or transient ischemic attack within 180 days of entry and insulin resistance without type 1 or type 2 diabetes.
Interventions: Pioglitazone or matching placebo.
Main outcomes and measures: A Cox proportional hazards regression model was created using baseline features to stratify patients above or below the median risk for stroke or MI within 5 years. Within each stratum, the efficacy of pioglitazone for preventing stroke or MI was calculated. Safety outcomes were death, heart failure, weight gain, and bone fracture.
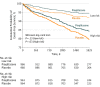
Results: Among 3876 participants (1338 women and 2538 men; mean [SD] age, 63 [11] years), the 5-year risk for stroke or MI was 6.0% in the pioglitazone group among patients at lower baseline risk compared with 7.9% in the placebo group (absolute risk difference, -1.9% [95% CI, -4.4% to 0.6%]). Among patients at higher risk, the risk was 14.7% in the pioglitazone group vs 19.6% for placebo (absolute risk difference, -4.9% [95% CI, -8.6% to 1.2%]). Hazard ratios were similar for patients below or above the median risk (0.77 vs 0.75; P = .92). Pioglitazone increased weight less among patients at higher risk but increased the risk for fracture more.
Conclusions and relevance: After an ischemic stroke or transient ischemic attack, patients at higher risk for stroke or MI derive a greater absolute benefit from pioglitazone compared with patients at lower risk. However, the risk for fracture is also higher.
Trial registration: clinicaltrials.gov Identifier: NCT00091949.
Conflict of interest statement
Figures
Comment in
-
Which Patients With Ischemic Stroke and Insulin Resistance May Benefit From Pioglitazone Hydrochloride?JAMA Neurol. 2017 Nov 1;74(11):1294-1296. doi: 10.1001/jamaneurol.2017.2142. JAMA Neurol. 2017. PMID: 28975238 No abstract available.
Similar articles
-
Pioglitazone Prevents Stroke in Patients With a Recent Transient Ischemic Attack or Ischemic Stroke: A Planned Secondary Analysis of the IRIS Trial (Insulin Resistance Intervention After Stroke).Circulation. 2018 Jan 30;137(5):455-463. doi: 10.1161/CIRCULATIONAHA.117.030458. Epub 2017 Oct 30. Circulation. 2018. PMID: 29084736 Clinical Trial.
-
Pioglitazone after Ischemic Stroke or Transient Ischemic Attack.N Engl J Med. 2016 Apr 7;374(14):1321-31. doi: 10.1056/NEJMoa1506930. Epub 2016 Feb 17. N Engl J Med. 2016. PMID: 26886418 Free PMC article. Clinical Trial.
-
Pioglitazone Therapy in Patients With Stroke and Prediabetes: A Post Hoc Analysis of the IRIS Randomized Clinical Trial.JAMA Neurol. 2019 May 1;76(5):526-535. doi: 10.1001/jamaneurol.2019.0079. JAMA Neurol. 2019. PMID: 30734043 Free PMC article. Clinical Trial.
-
Efficacy of lower doses of pioglitazone after stroke or transient ischaemic attack in patients with insulin resistance.Diabetes Obes Metab. 2022 Jun;24(6):1150-1158. doi: 10.1111/dom.14687. Epub 2022 Mar 18. Diabetes Obes Metab. 2022. PMID: 35253334 Review.
-
In praise of pioglitazone: An economically efficacious therapy for type 2 diabetes and other manifestations of the metabolic syndrome.Diabetes Obes Metab. 2023 Nov;25(11):3093-3102. doi: 10.1111/dom.15222. Epub 2023 Aug 3. Diabetes Obes Metab. 2023. PMID: 37534526 Review.
Cited by
-
Multi-Target Neuroprotection of Thiazolidinediones on Alzheimer's Disease via Neuroinflammation and Ferroptosis.J Alzheimers Dis. 2023;96(3):927-945. doi: 10.3233/JAD-230593. J Alzheimers Dis. 2023. PMID: 37927258 Free PMC article. Review.
-
Cardiovascular Outcomes in Patients With Previous Myocardial Infarction and Mild Diabetes Mellitus Following Treatment With Pioglitazone: Reports of a Randomised Trial From The Japan Working Group for the Assessment Whether Pioglitazone Protects DM Patients Against Re-Infarction (PPAR Study).EClinicalMedicine. 2018 Oct 22;4-5:10-24. doi: 10.1016/j.eclinm.2018.09.006. eCollection 2018 Oct-Nov. EClinicalMedicine. 2018. PMID: 31193597 Free PMC article.
-
Pioglitazone and barriers to effective post-stroke comorbidity management in stroke survivors with diabetes.Neurosciences (Riyadh). 2024 Jan;29(1):44-50. doi: 10.17712/nsj.2024.1.20230043. Neurosciences (Riyadh). 2024. PMID: 38195138 Free PMC article.
-
Effect of pioglitazone in acute ischemic stroke patients with diabetes mellitus: a nested case-control study.Cardiovasc Diabetol. 2019 May 31;18(1):67. doi: 10.1186/s12933-019-0874-5. Cardiovasc Diabetol. 2019. PMID: 31151454 Free PMC article.
-
Post-stroke cognitive impairment and the risk of stroke recurrence and death in patients with insulin resistance.J Stroke Cerebrovasc Dis. 2022 Oct;31(10):106744. doi: 10.1016/j.jstrokecerebrovasdis.2022.106744. Epub 2022 Aug 27. J Stroke Cerebrovasc Dis. 2022. PMID: 36037680 Free PMC article.
References
-
- Kernan WN, Ovbiagele B, Black HR, et al. ; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease . Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(7):2160-2236. - PubMed
-
- Semenkovich CF. Insulin resistance and a long, strange trip. N Engl J Med. 2016;374(14):1378-1379. - PubMed
-
- Davidoff F. Can knowledge about heterogeneity in treatment effects help us choose wisely? Ann Intern Med. 2017;166(2):141-142. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

