Population pharmacokinetics of treosulfan and development of a limited sampling strategy in children prior to hematopoietic stem cell transplantation
- PMID: 28975382
- PMCID: PMC5748442
- DOI: 10.1007/s00228-017-2344-x
Population pharmacokinetics of treosulfan and development of a limited sampling strategy in children prior to hematopoietic stem cell transplantation
Abstract
Purpose: There is an increasing interest in use of treosulfan (TREO), a structural analogue of busulfan, as an agent in conditioning regimens prior to hematopoietic stem cell transplantation (HSCT), both in pediatric and adult populations. The aim of this study was to develop a population pharmacokinetic model and to establish limited sampling strategies (LSSs) enabling accurate estimation of exposure to this drug.
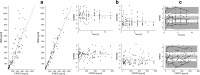
Methods: The study included 15 pediatric patients with malignant and non-malignant diseases, undergoing conditioning regimens prior to HSCT including TREO administered as a 1 h or 2 h infusion at daily doses of 10, 12, or 14 g/m2. A population pharmacokinetic model was developed by means of non-linear mixed-effect modeling approach in Monolix® software. Multivariate regression analysis and Bayesian method were used to develop 2- and 3-point strategies for estimation of exposure to TREO.
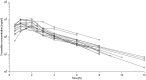
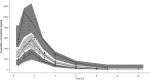
Results: Pharmacokinetics of TREO was best described with a two-compartmental linear model with proportional residual error. Following sampling schedules allowed accurate estimation of exposure to TREO: 1 h and 6 h or 1 h, 2 h, and 6 h for a TREO dose 12 g/m2 in a 1 h infusion, or at 2 h and 6 h or 2 h, 4 h, and 8 h for a TREO dose of 12 g/m2 and 14 g/m2 in a 2 h infusion.
Conclusions: A two-compartmental population pharmacokinetic model of TREO was developed and successfully used to establish 2- and 3-point LSSs for accurate and precise estimation of TREO AUC0→∞.
Keywords: Area under curve; Hematopoietic stem cell transplantation; Infusions, intravenous; Population pharmacokinetics.
Conflict of interest statement
Ethical approval
All procedures performed in study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Contributions of authors
D. Danielak supervised the study, performed modeling, validation, sampling strategy development and wrote the manuscript. J. Twardosz performed modeling and validation procedures. A. Kasprzyk developed the HPLC-MS/MS method and determined treosulfan concentrations. J. Wachowiak and K. Kałwak supervised patient recruitment and critically reviewed the manuscript. F. Główka supervised the study and critically reviewed the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Population pharmacokinetics of treosulfan in paediatric patients undergoing hematopoietic stem cell transplantation.Br J Clin Pharmacol. 2019 Sep;85(9):2033-2044. doi: 10.1111/bcp.13995. Epub 2019 Jul 1. Br J Clin Pharmacol. 2019. PMID: 31144349 Free PMC article.
-
Relationship between exposure to treosulfan and its monoepoxytransformer - An insight from population pharmacokinetic study in pediatric patients before hematopoietic stem cell transplantation.Eur J Pharm Sci. 2018 Jul 30;120:1-9. doi: 10.1016/j.ejps.2018.04.036. Epub 2018 Apr 27. Eur J Pharm Sci. 2018. PMID: 29705215
-
Pharmacokinetics of treosulfan and its active monoepoxide in pediatric patients after intravenous infusion of high-dose treosulfan prior to HSCT.Eur J Pharm Sci. 2015 Feb 20;68:87-93. doi: 10.1016/j.ejps.2014.12.010. Epub 2014 Dec 16. Eur J Pharm Sci. 2015. PMID: 25527118 Clinical Trial.
-
High-dose treosulfan in conditioning prior to hematopoietic stem cell transplantation.Expert Opin Investig Drugs. 2010 Oct;19(10):1275-95. doi: 10.1517/13543784.2010.517744. Expert Opin Investig Drugs. 2010. PMID: 20836619 Review.
-
Pharmacokinetics of high-dose i.v. treosulfan in children undergoing treosulfan-based preparative regimen for allogeneic haematopoietic SCT.Bone Marrow Transplant. 2008 Oct;42 Suppl 2:S67-70. doi: 10.1038/bmt.2008.287. Bone Marrow Transplant. 2008. PMID: 18978748 Review.
Cited by
-
Determinants of Interpatient Variability in Treosulfan Pharmacokinetics in AML Patients Undergoing Autologous Stem Cell Transplantation.Int J Mol Sci. 2024 Jul 27;25(15):8215. doi: 10.3390/ijms25158215. Int J Mol Sci. 2024. PMID: 39125785 Free PMC article.
-
Population pharmacokinetic approach for evaluation of treosulfan and its active monoepoxide disposition in plasma and brain on the basis of a rat model.Pharmacol Rep. 2020 Oct;72(5):1297-1309. doi: 10.1007/s43440-020-00115-0. Epub 2020 May 30. Pharmacol Rep. 2020. PMID: 32474888 Free PMC article.
-
Modelling of neutrophil dynamics in children receiving busulfan or treosulfan for haematopoietic stem cell transplant conditioning.Br J Clin Pharmacol. 2020 Aug;86(8):1537-1549. doi: 10.1111/bcp.14260. Epub 2020 Mar 3. Br J Clin Pharmacol. 2020. PMID: 32077123 Free PMC article.
-
Leveraging machine learning in limited sampling strategies for efficient estimation of the area under the curve in pharmacokinetic analysis: a review.Eur J Clin Pharmacol. 2025 Feb;81(2):183-201. doi: 10.1007/s00228-024-03780-9. Epub 2024 Nov 21. Eur J Clin Pharmacol. 2025. PMID: 39570408 Review.
-
Population pharmacokinetics of treosulfan in paediatric patients undergoing hematopoietic stem cell transplantation.Br J Clin Pharmacol. 2019 Sep;85(9):2033-2044. doi: 10.1111/bcp.13995. Epub 2019 Jul 1. Br J Clin Pharmacol. 2019. PMID: 31144349 Free PMC article.
References
-
- Ljungman P, Bregni M, Brune M, Cornelissen J, de WT, Dini G, Einsele H, Gaspar HB, Gratwohl A, Passweg J, Peters C, Rocha V, Saccardi R, Schouten H, Sureda A, Tichelli A, Velardi A, Niederwieser D. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe 2009. Bone Marrow Transplant. 2009;45:219–234. doi: 10.1038/bmt.2009.141. - DOI - PubMed
-
- Wachowiak J, Sykora KW, Cornish J, Chybicka A, Kowalczyk JR, Gorczynska E, Choma M, Grund G, Peters C. Treosulfan-based preparative regimens for allo-HSCT in childhood hematological malignancies: a retrospective study on behalf of the EBMT Pediatric Diseases Working Party. Bone Marrow Transplant. 2011;46:1510–1518. doi: 10.1038/bmt.2010.343. - DOI - PubMed
-
- Slatter MA, Boztug H, Pötschger U, Sykora K-W, Lankester A, Yaniv I, Sedlacek P, Glogova E, Veys P, Gennery AR, Peters C, Inborn Errors EBMT, Parties PDW. Treosulfan-based conditioning regimens for allogeneic haematopoietic stem cell transplantation in children with non-malignant diseases. Bone Marrow Transplant. 2015;50:1536–1541. doi: 10.1038/bmt.2015.171. - DOI - PubMed
-
- Morillo-Gutierrez B, Beier R, Rao K, Burroughs L, Schulz A, Ewins A-M, Gibson B, Sedlacek P, Krol L, Strahm B, Zaidman I, Kalwak K, Talano J-A, Woolfrey A, Fraser C, Meyts I, Müller I, Wachowiak J, Bernardo ME, Veys P, Sykora K-W, Gennery AR, Slatter M. Treosulfan-based conditioning for allogeneic HSCT in children with chronic granulomatous disease: a multicenter experience. Blood. 2016;128:440–448. doi: 10.1182/blood-2016-03-704015. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

