An Algorithm to Identify Compounded Non-Sterile Products that Can Be Formulated on a Commercial Scale or Imported to Promote Safer Medication Use in Children
- PMID: 28975916
- PMCID: PMC5597107
- DOI: 10.3390/pharmacy3040284
An Algorithm to Identify Compounded Non-Sterile Products that Can Be Formulated on a Commercial Scale or Imported to Promote Safer Medication Use in Children
Abstract
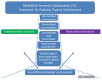
The lack of commercially-available pediatric drug products and dosage forms is well-known. A group of clinicians and scientists with a common interest in pediatric drug development and medicines-use systems developed a practical framework for identifying a list of active pharmaceutical ingredients (APIs) with the greatest market potential for development to use in pediatric patients. Reliable and reproducible evidence-based drug formulations designed for use in pediatric patients are needed vitally, otherwise safe and consistent clinical practices and outcomes assessments will continue to be difficult to ascertain. Identification of a prioritized list of candidate APIs for oral formulation using the described algorithm provides a broader integrated clinical, scientific, regulatory, and market basis to allow for more reliable dosage forms and safer, effective medicines use in children of all ages. Group members derived a list of candidate API molecules by factoring in a number of pharmacotherapeutic, scientific, manufacturing, and regulatory variables into the selection algorithm that were absent in other rubrics. These additions will assist in identifying and categorizing prime API candidates suitable for oral formulation development. Moreover, the developed algorithm aids in prioritizing useful APIs with finished oral liquid dosage forms available from other countries with direct importation opportunities to North America and beyond.
Keywords: compounded medications; formulation development; patient safety; pediatric.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Compounded Nonsterile Preparations and FDA-Approved Commercially Available Liquid Products for Children: A North American Update.Pharmaceutics. 2022 May 10;14(5):1032. doi: 10.3390/pharmaceutics14051032. Pharmaceutics. 2022. PMID: 35631618 Free PMC article.
-
Preparation and Physicochemical Stability of Liquid Oral Dosage Forms Free of Potentially Harmful Excipient Designed for Pediatric Patients.Pharmaceutics. 2019 Apr 18;11(4):190. doi: 10.3390/pharmaceutics11040190. Pharmaceutics. 2019. PMID: 31003500 Free PMC article.
-
Providing Suitable Pediatric Formulations for Canadian Children: A Call for Action.Can J Hosp Pharm. 2020 Fall;73(4):247-256. Epub 2020 Oct 1. Can J Hosp Pharm. 2020. PMID: 33100356 Free PMC article.
-
Availability of Authorizations from EMA and FDA for Age-Appropriate Medicines Contained in the WHO Essential Medicines List for Children 2019.Pharmaceutics. 2020 Apr 1;12(4):316. doi: 10.3390/pharmaceutics12040316. Pharmaceutics. 2020. PMID: 32244848 Free PMC article. Review.
-
Patient safety issues associated with the use of compounded medicines as alternatives to approved pharmaceutical products in Europe and how best practice can improve outcomes.Int J Risk Saf Med. 2020;31(3):133-144. doi: 10.3233/JRS-200002. Int J Risk Saf Med. 2020. PMID: 32538874 Review.
Cited by
-
Where are the Ambulatory Care Pediatric Pharmacists?Children (Basel). 2019 Feb 7;6(2):25. doi: 10.3390/children6020025. Children (Basel). 2019. PMID: 30736468 Free PMC article.
-
Drug Prescriptions Requiring Compounding at a Canadian University Affiliated Pediatric Hospital: A Cross-Sectional Study.Children (Basel). 2023 Jan 11;10(1):147. doi: 10.3390/children10010147. Children (Basel). 2023. PMID: 36670697 Free PMC article.
-
Positioning a Paediatric Compounded Non-Sterile Product Electronic Repository (pCNPeRx) within the Health Information Technology Infrastructure.Pharmacy (Basel). 2015 Dec 24;4(1):2. doi: 10.3390/pharmacy4010002. Pharmacy (Basel). 2015. PMID: 28970375 Free PMC article.
-
Extemporaneous Compounding: Selective Pharmacists with Separate Skill.Innov Pharm. 2019 Oct 31;10(4):10.24926/iip.v10i4.1660. doi: 10.24926/iip.v10i4.1660. eCollection 2019. Innov Pharm. 2019. Retraction in: Innov Pharm. 2020 Feb 25;11(1). doi: 10.24926/iip.v11i1.3942. PMID: 34007590 Free PMC article. Retracted. Review.
References
-
- Committee on Drugs Off-label use of drugs in children. Pediatrics. 2014;133:563–567. - PubMed
-
- Compounding Compendium. The United States Pharmacopeial Convention; Rockville, MD, USA: [(accessed on 21 August 2015)]. Available online: http://www.usp.org/sites/default/files/usp_pdf/EN/products/2015_usp_comp....
-
- Jew R.K., Soo-Hoo W., Erush S.C. Extemporaneous Formulations for Pediatric, Geriatric, and Special Needs Patients. 2nd ed. American Society of Health-System Pharmacists; Bethesda, MD, USA: 2010.
LinkOut - more resources
Full Text Sources
Other Literature Sources