The Efficiency of Bone Marrow Aspiration for the Harvest of Connective Tissue Progenitors from the Human Iliac Crest
- PMID: 28976432
- PMCID: PMC5621565
- DOI: 10.2106/JBJS.17.00094
The Efficiency of Bone Marrow Aspiration for the Harvest of Connective Tissue Progenitors from the Human Iliac Crest
Abstract
Background: The rational design and optimization of tissue engineering strategies for cell-based therapy requires a baseline understanding of the concentration and prevalence of osteogenic progenitor cell populations in the source tissues. The aim of this study was to (1) define the efficiency of, and variation among individuals in, bone marrow aspiration as a means of osteogenic connective tissue progenitor (CTP-O) harvest compared with harvest from iliac cancellous bone, and (2) determine the location of CTP-Os within native cancellous bone and their distribution between the marrow-space and trabecular-surface tissue compartments.
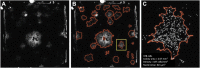
Methods: Eight 2-mL bone marrow aspiration (BMA) samples and one 7-mm transcortical biopsy sample were obtained from the anterior iliac crest of 33 human subjects. Two cell populations were obtained from the iliac cancellous bone (ICB) sample. The ICB sample was placed into αMEM (alpha-minimal essential medium) with antibiotic-antimycotic and minced into small pieces (1 to 2 mm in diameter) with a sharp osteotome. Cells that could be mechanically disassociated from the ICB sample were defined as marrow-space (IC-MS) cells, and cells that were disassociated only after enzymatic digestion were defined as trabecular-surface (IC-TS) cells. The 3 sources of bone and marrow-derived cells were compared on the basis of cellularity and the concentration and prevalence of CTP-Os through colony-forming unit (CFU) analysis.
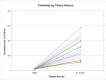
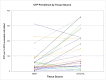
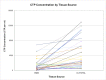
Results: Large variation was seen among patients with respect to cell and CTP-O yield from the IC-MS, IC-TS, and BMA samples and in the relative distribution of CTP-Os between the IC-MS and IC-TS fractions. The CTP-O prevalence was highest in the IC-TS fraction, which was 11.4-fold greater than in the IC-MS fraction (p < 0.0001) and 1.7-fold greater than in the BMA fraction. However, the median concentration of CTP-Os in the ICB (combining MS and TS fractions) was only 3.04 ± 1.1-fold greater than that in BMA (4,265 compared with 1,402 CTP/mL; p = 0.00004).
Conclusions: Bone marrow aspiration of a 2-mL volume at a given needle site is an effective means of harvesting CTP-Os, albeit diluted with peripheral blood. However, the median concentration of CTP-Os is 3-fold less than from native iliac cancellous bone. The distribution of CTP-Os between the IC-MS and IC-TS fractions varies widely among patients.
Clinical relevance: Bone marrow aspiration is an effective means of harvesting CTP-Os but is associated with dilution with peripheral blood. Overall, we found that 63.5% of all CTP-Os within iliac cancellous bone resided on the trabecular surface; however, 48% of the patients had more CTP-Os contributed by the IC-MS than the IC-TS fraction.
Figures



References
-
- Muschler GF, Midura RJ. Connective tissue progenitors: practical concepts for clinical applications. Clin Orthop Relat Res. 2002. February;395:66-80. - PubMed
-
- Chahla J, Piuzzi NS, Mitchell JJ, Dean CS, Pascual-Garrido C, LaPrade RF, Muschler GF. Intra-articular cellular therapy for osteoarthritis and focal cartilage defects of the knee: a systematic review of the literature and study quality analysis. J Bone Joint Surg Am. 2016. September 21;98(18):1511-21. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

