Adjuvant Chemoradiotherapy With Epirubicin, Cisplatin, and Fluorouracil Compared With Adjuvant Chemoradiotherapy With Fluorouracil and Leucovorin After Curative Resection of Gastric Cancer: Results From CALGB 80101 (Alliance)
- PMID: 28976791
- PMCID: PMC5678342
- DOI: 10.1200/JCO.2017.74.2130
Adjuvant Chemoradiotherapy With Epirubicin, Cisplatin, and Fluorouracil Compared With Adjuvant Chemoradiotherapy With Fluorouracil and Leucovorin After Curative Resection of Gastric Cancer: Results From CALGB 80101 (Alliance)
Abstract
Purpose After curative resection of gastric or gastroesophageal junction adenocarcinoma, Intergroup Trial 0116 (Phase III trial of postoperative adjuvant radiochemotherapy for high risk gastric and gastroesophageal junction adenocarcinoma: Demonstrated superior survival for patients who received postoperative chemoradiotherapy with bolus fluorouracil (FU) and leucovorin (LV) compared with surgery alone. CALGB 80101 (Alliance; Phase III Intergroup Trial of Adjuvant Chemoradiation After Resection of Gastric or Gastroesophageal Adenocarcinoma) assessed whether a postoperative chemoradiotherapy regimen that replaced FU plus LV with a potentially more active systemic therapy could further improve overall survival. Patients and Methods Between April 2002 and May 2009, 546 patients who had undergone a curative resection of stage IB through IV (M0) gastric or gastroesophageal junction adenocarcinoma were randomly assigned to receive either postoperative FU plus LV before and after combined FU and radiotherapy (FU plus LV arm) or postoperative epirubicin, cisplatin, and infusional FU (ECF) before and after combined FU and radiotherapy (ECF arm). Results With a median follow-up duration of 6.5 years, 5-year overall survival rates were 44% in the FU plus LV arm and 44% in the ECF arm ( Plogrank = .69; multivariable hazard ratio, 0.98; 95% CI, 0.78 to 1.24 comparing ECF with FU plus LV). Five-year disease-free survival rates were 39% in the FU plus LV arm and 37% in the ECF arm ( Plogrank = .94; multivariable hazard ratio, 0.96; 95% CI, 0.77 to 1.20). In post hoc analyses, the effect of treatment seemed to be similar across all examined patient subgroups. Conclusion After a curative resection of gastric or gastroesophageal junction adenocarcinoma, postoperative chemoradiotherapy using a multiagent regimen of ECF before and after radiotherapy does not improve survival compared with standard FU and LV before and after radiotherapy.
Figures

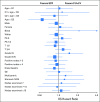

Comment in
-
Reply to L. Fornaro et al.J Clin Oncol. 2018 Apr 10;36(11):1179-1180. doi: 10.1200/JCO.2017.77.1030. Epub 2018 Feb 28. J Clin Oncol. 2018. PMID: 29489430 No abstract available.
-
CALGB 80101 and the Final Call for Preoperative Chemotherapy in Gastric Cancer.J Clin Oncol. 2018 Apr 10;36(11):1178-1179. doi: 10.1200/JCO.2017.76.6493. Epub 2018 Feb 28. J Clin Oncol. 2018. PMID: 29489431 No abstract available.
-
Gastrointestinal Cancers-Carving Out the Optimal Local Therapies in the Gastrointestinal Tract.Int J Radiat Oncol Biol Phys. 2018 Oct 1;102(2):233-242. doi: 10.1016/j.ijrobp.2018.05.026. Int J Radiat Oncol Biol Phys. 2018. PMID: 30191854 Free PMC article. No abstract available.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. : Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359-E386, 2015 - PubMed
-
- http://seer.cancer.gov/csr/1975_2012/ National Cancer Institute: Previous Version: SEER Cancer Statistics Review, 1975-2012.
-
- Macdonald JS, Smalley SR, Benedetti J, et al. : Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 345:725-730, 2001 - PubMed
-
- Cunningham D, Allum WH, Stenning SP, et al. : Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 355:11-20, 2006 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA149222/CA/NCI NIH HHS/United States
- U10 CA031946/CA/NCI NIH HHS/United States
- U10 CA033601/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA138561/CA/NCI NIH HHS/United States
- U10 CA180868/CA/NCI NIH HHS/United States
- U10 CA047577/CA/NCI NIH HHS/United States
- U10 CA032291/CA/NCI NIH HHS/United States
- R01 CA118553/CA/NCI NIH HHS/United States
- U10 CA077658/CA/NCI NIH HHS/United States
- UL1 TR001863/TR/NCATS NIH HHS/United States
- K12 CA090625/CA/NCI NIH HHS/United States
- P30 CA086862/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- U10 CA180791/CA/NCI NIH HHS/United States
- U10 CA180857/CA/NCI NIH HHS/United States
- U10 CA180850/CA/NCI NIH HHS/United States
- U10 CA077651/CA/NCI NIH HHS/United States
- U10 CA180790/CA/NCI NIH HHS/United States
- R01 CA169141/CA/NCI NIH HHS/United States
- U10 CA180867/CA/NCI NIH HHS/United States
- U10 CA180838/CA/NCI NIH HHS/United States
- U10 CA047642/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- P50 CA127003/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

