Protection of bats (Eptesicus fuscus) against rabies following topical or oronasal exposure to a recombinant raccoon poxvirus vaccine
- PMID: 28976983
- PMCID: PMC5643138
- DOI: 10.1371/journal.pntd.0005958
Protection of bats (Eptesicus fuscus) against rabies following topical or oronasal exposure to a recombinant raccoon poxvirus vaccine
Abstract
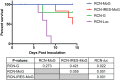
Rabies is an ancient neglected tropical disease that causes tens of thousands of human deaths and millions of cattle deaths annually. In order to develop a new vaccine for potential use in bats, a reservoir of rabies infection for humans and animals alike, an in silico antigen designer tool was used to create a mosaic glycoprotein (MoG) gene using available sequences from the rabies Phylogroup I glycoprotein. This sequence, which represents strains more likely to occur in bats, was cloned into raccoonpox virus (RCN) and the efficacy of this novel RCN-MoG vaccine was compared to RCN-G that expresses the glycoprotein gene from CVS-11 rabies or luciferase (RCN-luc, negative control) in mice and big brown bats (Eptesicus fuscus). Mice vaccinated and boosted intradermally with 1 x 107 plaque forming units (PFU) of each RCN-rabies vaccine construct developed neutralizing antibodies and survived at significantly higher rates than controls. No significant difference in antibody titers or survival was noted between rabies-vaccinated groups. Bats were vaccinated either oronasally (RCN-G, RCN-MoG) with 5x107 PFU or by topical application in glycerin jelly (RCN-MoG, dose 2x108 PFU), boosted (same dose and route) at 46 days post vaccination (dpv), and then challenged with wild-type big brown variant RABV at 65 dpv. Prior to challenge, 90% of RCN-G and 75% of RCN-MoG oronasally vaccinated bats had detectable levels of serum rabies neutralizing antibodies. Bats from the RCN-luc and topically vaccinated RCN-MoG groups did not have measurable antibody responses. The RCN-rabies constructs were highly protective and not significantly different from each other. RCN-MoG provided 100% protection (n = 9) when delivered oronasally and 83% protection (n = 6) when delivered topically; protection provided by the RCN-G construct was 70% (n = 10). All rabies-vaccinated bats survived at a significantly (P ≤ 0.02) higher rate than control bats (12%; n = 8). We have demonstrated the efficacy of a novel, in silico designed rabies MoG antigen that conferred protection from rabies challenge in mice and big brown bats in laboratory studies. With further development, topical or oronasal administration of the RCN-MoG vaccine could potentially mitigate rabies in wild bat populations, reducing spillover of this deadly disease into humans, domestic mammals, and other wildlife.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Wunner WWH, Conzelmann K-K. Rabies Virus In: Jackson AC, editor. Rabies: Scientific Basis of the Disease and its Management. Third Oxford: Elsevier; 2013. pp. 17–49.
-
- WHO. Rabies: fact sheet [Internet]. http://www.who.int/mediacentre/factsheets/fs099/en/: WHO; 2016. http://www.who.int/mediacentre/factsheets/fs099/en/
-
- Johnson N, Aréchiga-Ceballos N, Aguilar-Setien A. Vampire Bat Rabies: Ecology, Epidemiology and Control. Viruses. 2014. pp. 1911–1928. doi: 10.3390/v6051911 - DOI - PMC - PubMed
-
- Ellison JA, Gilbert AT, Recuenco S, Moran D, Alvarez DA, Kuzmina N, et al. Bat Rabies in Guatemala. PLoS Negl Trop Dis. 2014;8 doi: 10.1371/journal.pntd.0003070 - DOI - PMC - PubMed
-
- Lee DN, Papeş M, Van den Bussche RA. Present and potential future distribution of common vampire bats in the Americas and the associated risk to cattle. PLoS One. 2012;7: e42466 doi: 10.1371/journal.pone.0042466 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

